Assessment of Microbial Load in Fresh and Frozen Broiler Chicken Samples from Lahore
Abstract
1. Introduction
Meat is a valuable and easily accessible source of high-quality animal protein for consumers. Among essential foods, it ranks as one of the most consumed due to its nutrient-rich profile, which also provides a conducive environment for microbial growth [1]. In Pakistan, broiler meat production has shown significant growth, reaching 587,000 metric tons in 2010 [2]. From 1994-1995 to 2015-2016, the annual average growth rates of meat production and exports were reported at 3% and 32%, respectively. Projections based on these trends estimate that by 2029-2030, total meat production in Pakistan could reach 6,078 thousand tons, with exports valued at $17,477 million [3]. However, concerns regarding the microbiological quality of chicken meat persist, as contamination during processing is a significant challenge [4].
Broiler chicken meat is a crucial dietary component, rich in essential vitamins such as A, D, and E [5]. However, chicken carcasses are frequently contaminated with pathogenic microorganisms like Salmonella spp., Listeria monocytogenes, and Escherichia coli O157:H7, which are associated with foodborne illnesses, including diarrhea and abdominal pain [6]. Studies indicate that E. coli, Salmonella, and Listeria are major contributors to meat-related illnesses, with E. coli accounting for up to 90% of these cases. These pathogens have also been detected in various food products, including raw and cooked poultry, seafood, shawarma, sandwiches, ready-to-eat (RTE) salads, mayonnaise, and even on the hands of food handlers, highlighting significant food safety concerns [7].
In Pakistan, foodborne diseases such as salmonellosis, shigellosis, campylobacteriosis, cholera, gastroenteritis, and brucellosis are widely recognized. RTE foods are often contaminated with pathogens like Campylobacter, Staphylococcus, Salmonella typhimurium, Escherichia coli O157:H7, and Listeria monocytogenes. Inadequate sanitation and hygiene practices exacerbate these risks, making foodborne illnesses a serious public health issue, particularly diarrhea, which disproportionately affects children and is a major health concern in the country [8].
Raw chicken is highly susceptible to contamination, harboring microorganisms on its feathers, skin, and intestinal tract. During slaughter, defeathering, evisceration, storage, and distribution, bacterial contamination becomes inevitable. Boneless poultry products, while versatile, are also prone to microbial spoilage if proper hygiene and handling practices are not followed [9]. Without stringent controls, microbial contamination can result in foodborne illnesses, posing a significant public health risk to consumers. Proper hygiene, storage, and cooking practices are crucial in mitigating these risks [10].
The objective of the current study was to assess the microbial load in fresh and frozen broiler chicken samples collected from various sources at selected sale points in the Lahore market. This study aimed to evaluate and compare the microbiological quality of chicken meat from different outlets, highlighting potential public health risks and the need for improved hygiene and handling practices in the supply chain.
2. Materials and Methods
2.1. Study Design and Sampling
This study was conducted in the Food Science and Technology Laboratory (Lab No. 102) at the University Institute of Diet and Nutritional Sciences (UIDNS), The University of Lahore. The investigation focused on comparing the quality standards of frozen chicken meat versus fresh local chicken. Specifically, the study evaluated the total bacterial count (TBC), Escherichia coli O157, fecal coliform, Salmonella, and Listeria, along with pH and color characteristics as indicators of microbial quality and meat freshness.
A total of 60 chicken samples were collected, comprising both frozen and fresh meat. The frozen samples were sourced from supermarket chains, while the fresh samples were obtained from local markets in Lahore. For sampling, the city of Lahore was divided into six zones: Gawalmandi (Zone A), DHA-1 (Zone B), Model Town (Zone C), Bhati Gate (Zone D), Kot Abdul Malik (Zone E), and Bahria Town (Zone F). From each zone, five frozen and five fresh chicken samples were collected to ensure a comprehensive assessment of quality standards across different sale points in the city.
2.2. Sample Inclusion and Exclusion Criteria
Samples of industrially processed broiler chicken meat were included only if they were properly sealed and stored at -18°C. Samples from open vendors were included if they were fresh and unpacked. These samples were immediately packed in sanitized polyethylene bags upon collection. Frozen samples with ruptured packaging or those subjected to temperature abuse were excluded. Fresh samples that were old, slaughtered earlier, or pre-packed were not included.
2.3. pH Analysis
The pH of chicken meat samples was measured using a digital pH meter (InoLab 720, Germany). The meter was calibrated with standard buffer solutions of pH 7.0 and 4.0 prior to use. The pH measurement was conducted by directly inserting the electrode into the meat samples following the protocol described by others. [11].
2.4. Color Analysis
The color of chicken meat was assessed instrumentally using a tristimulus colorimeter (CIELAB SPACE, Color Tech-PCM, USA). The instrument was calibrated with standard CTn values of 151 (lightness) and 54 (darkness). Samples were analyzed using a photocell to determine color values in the CIE Lab Cartesian coordinate system for lightness (L*), redness (a*), and yellowness (b*), following methods described earlier [11. 12].
2.5. Microbial Assay
The microbiological quality of chicken meat was assessed by determining the total bacterial count (TBC), fecal coliform (FC) count, Escherichia coli O157 (EC) count, Listeria spp. and Salmonella spp. Samples were plated on selective growth media, including MacConkey agar, Plate Count Agar, and XLT4 (Xylose Lysine Tergitol 4) agar, to enumerate specific pathogens [13, 14].
Total Bacterial Count (TBC): The microbiological status of fresh and frozen chicken meat was assessed using a 25 g sample diluted in 225 ml of BPW for a 10-1 serial dilution. Pathogens such as Enterobacteria, Bacillus cereus, and Staphylococci were analyzed using standard techniques [15].
E. coli O157: A 25 g sample was diluted in 225 ml of Buffered Peptone Water (BPW) to prepare a 10-1 serial dilution. The dilution was plated on Eosin Methylene Blue (EMB) agar and incubated overnight at 37°C for 24 hours. Colonies were counted and expressed as CFU/ml [16].
Fecal Coliform (FC): The 25 g diluted sample was plated on MacConkey agar and incubated at 37°C for 24 hours. Colony counts were expressed as CFU/ml [16].
Salmonella species: A 25 g meat sample diluted in 225 ml of BPW to prepare a 10-1 serial dilution. The ISO6579 protocol was followed, including serotyping and phage typing to determine contamination levels. Studies from public health laboratories were referenced for baseline contamination rates [17].
Listeria species: Samples were diluted as mentioned above. Different Listeria species, including L. monocytogenes, L. welshimeri, L. innocua, and L. seeligeri, were detected. Studies were aligned with findings from retail chicken surveys in South Africa, indicating contamination of both fresh and frozen chicken meat [18, 19].
2.6. Statistical Analyses
Data were analyzed using the GLM Procedure of SAS (SAS for Academics) under analysis of variance (ANOVA). The sample collection points defined as zones and the frozen vs fresh meat were treated as a fixed effect. Results were considered statistically significant at p ≤ 0.05.
3. Results and Discussion
3.1. pH of Meat
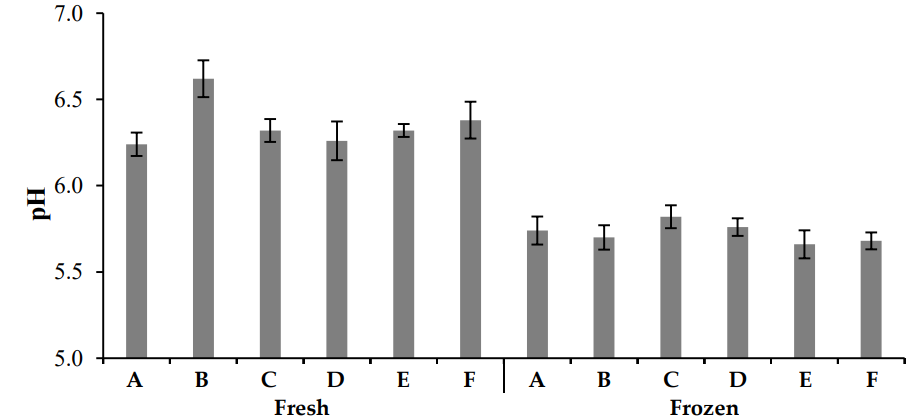
Figure 1 presents the pH levels in fresh and frozen chicken meat. Results indicated significant differences between fresh and frozen chicken samples as well as between different zones. The highest pH value (5.82) in fresh chicken was recorded in Zone C, followed by Zones D, A, B, F, and E. In frozen chicken, the highest pH value (6.62) was observed in Zone B, followed by Zones F, E, C, D, and A. The means of pH within the fresh and frozen groups were significantly different, while there were no significant differences within each group. The pH of meat plays a crucial role in its water-holding capacity. Meat with lower pH has reduced water retention compared to meat samples with normal or high pH, which can lead to higher cooking losses and protein denaturation [20, 21].
3.2. Color Analysis
In this study, chicken meat color was evaluated using a colorimeter, which measured the L* (brightness), a* (redness), and b* (yellowness) values. The results (Figures 2–4) revealed significant differences in color parameters for both fresh and frozen chicken samples. Fresh chicken samples exhibited higher L* (brightness) values, ranging from 56.64 ± 0.693 to 50.42 ± 0.871, compared to frozen chicken, which showed lower L* values (44.46 ± 1.456 to 41.26 ± 1.603). For a*, fresh chicken samples ranged from 4.6 ± 0.442 to 8.0 ± 0.131, while frozen chicken samples ranged from 7.6 ± 0.584 to 13.4 ± 0.806. The greatest a* value was recorded in Zone A of fresh chicken (12.25 ± 0.662), while the highest value in frozen chicken was found in Zone B (15.52 ± 0.432). Regarding b*, fresh chicken samples ranged from 5.6 ± 0.765 to 7.60 ± 0.654, while frozen chicken samples ranged from 7.2 ± 0.463 to 10.6 ± 0.543. The highest b* value was observed in Zone A for fresh chicken (12.24 ± 0.512) and in Zone B for frozen chicken (13.22 ± 0.234). These results indicate significant differences in color characteristics, with fresh chicken showing higher brightness (L*), while frozen chicken exhibited more redness (a*) and yellowness (b*).
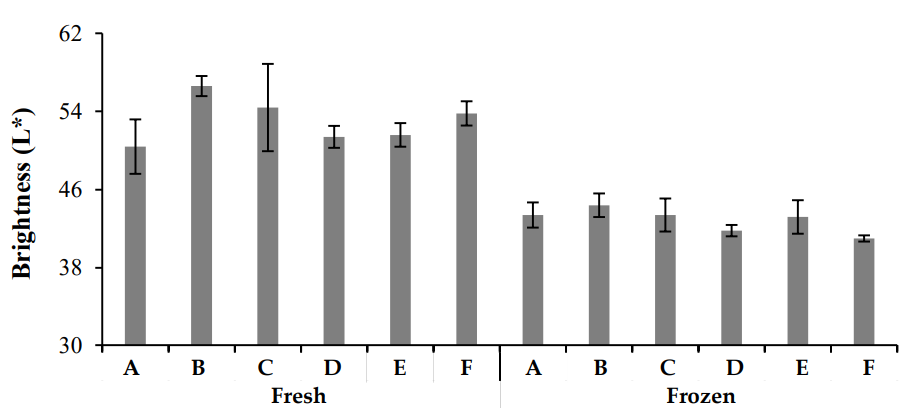
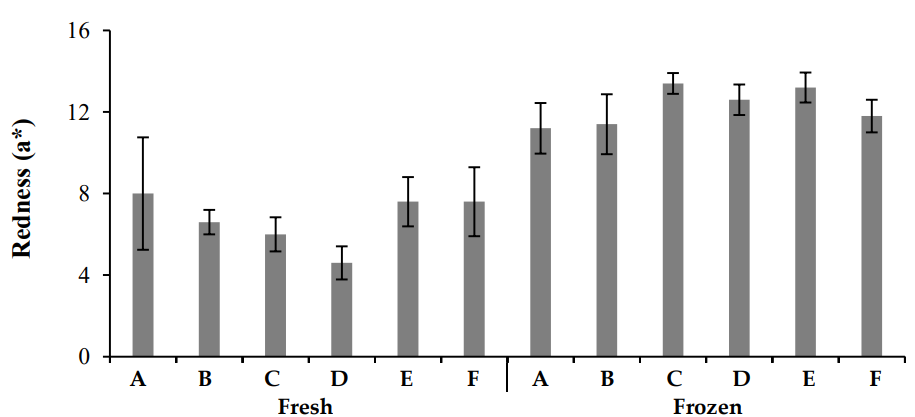
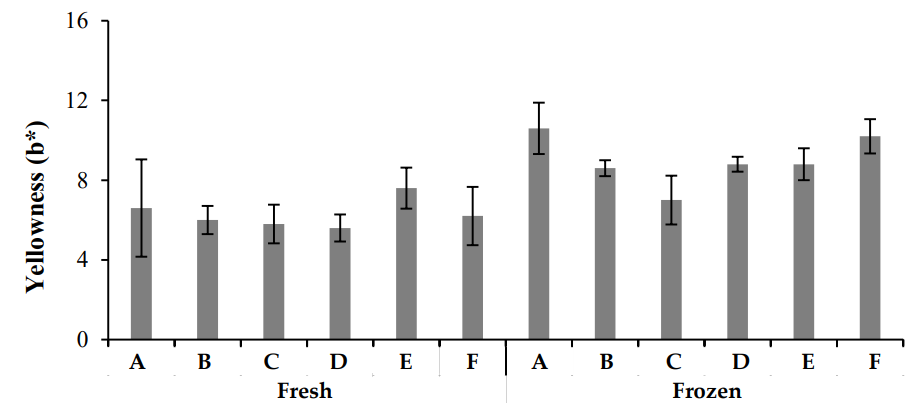
Color variations in meat are primarily due to myoglobin oxidation and protein denaturation, both of which are influenced by pH. As pH decreases, meat color becomes paler, whereas higher pH results in a more red color. The pinkish-red color of meat is due to myoglobin oxidation, which then turns into oxymyoglobin and eventually into metmyoglobin, giving the meat a brownish hue [22]. The oxidation of myoglobin is reversible.
3.3. Microbial Assay
3.3.1 E. coli Count
In this study, both fresh and frozen chicken meat samples from various zones in Lahore were tested for E. coli O157:H7. The results showed that no E. coli O157:H7 was detected in any of the frozen chicken samples. In contrast, fresh chicken samples from Zones A, D, and E were found to be positive for E. coli O157:H7, although other fresh samples were negative. The results (Figure 5) showed that E. coli O157:H7 was not detected in frozen chicken and was only present in certain fresh chicken samples.
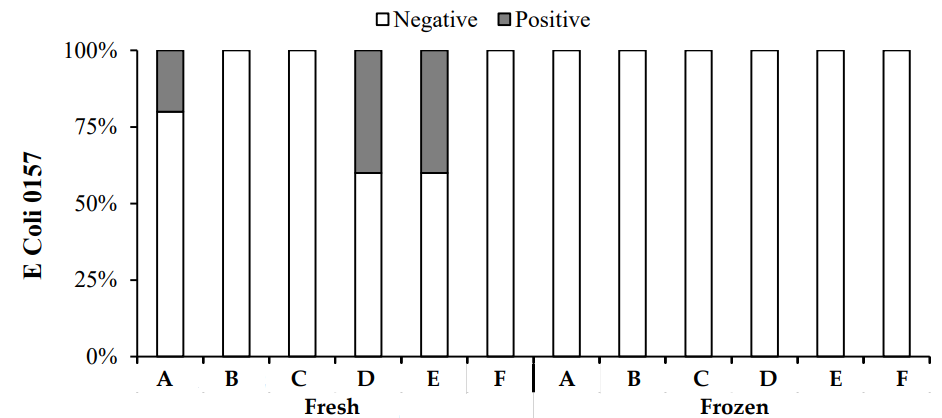
E. coli is a key indicator of meat quality and safety [23, 24]. E. coli O157:H7 is a particularly concerning strain, often associated with meat and linked to several outbreaks globally. For instance, an outbreak in the USA in 2002 was traced to contaminated beef, resulting in the hospitalization of several individuals [25, 26].
3.3.2 Salmonella
In this study, the presence of Salmonella in fresh and frozen chicken samples was assessed using traditional methods. Figure 6 presents the results, which showed that Salmonella was detected in some fresh chicken samples. Specifically, all samples from Zone A of fresh chicken were positive for Salmonella, and some samples from Zones B, D, and E also tested positive. However, all frozen chicken samples from the six zones tested negative for Salmonella, except for a few positive samples in Zone A. Salmonella is one of the most common foodborne pathogens, with many outbreaks linked to meat products [27].
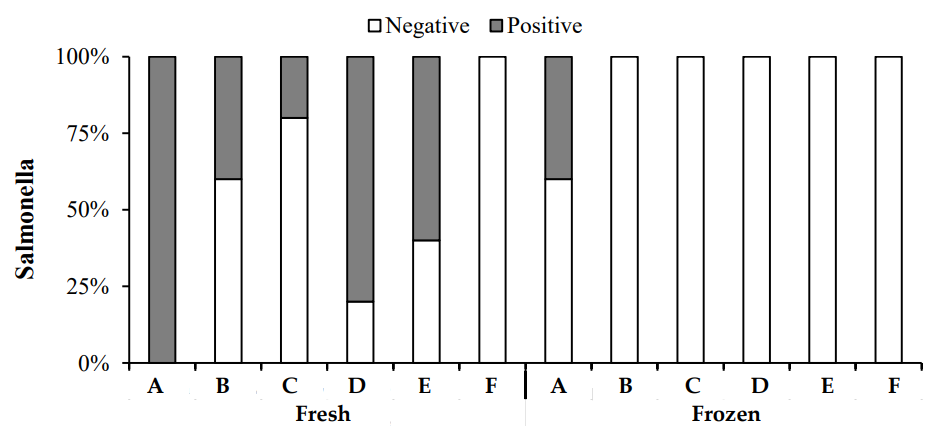
3.3.3 Listeria
In this study, Listeria was tested in both fresh and frozen chicken samples. The results showed that Listeria was not detected in most samples, with only one fresh sample from Zone D testing positive (Figure 7). Overall, the prevalence of Listeria in both fresh and frozen chicken samples was low. Listeria monocytogenes is a significant pathogen in meat products due to its ability to grow under refrigerated conditions, making it a concern for both fresh and frozen meat [28].
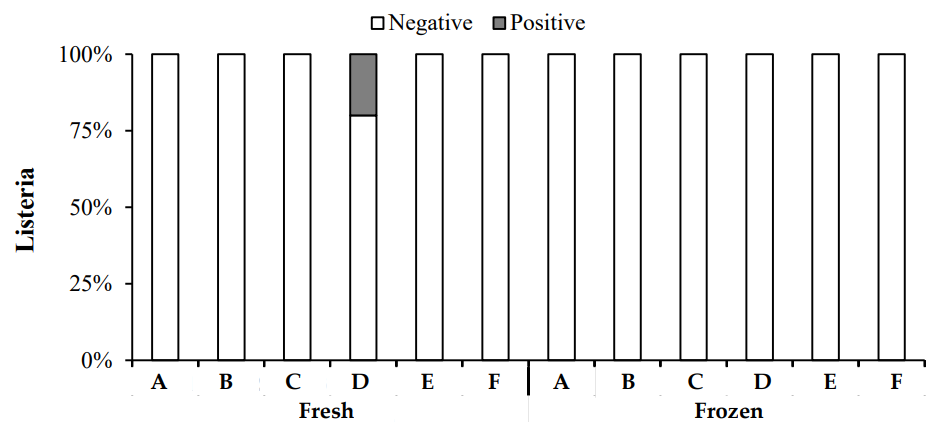
3.3.4 Fecal Coliform
The results of fecal coliform testing (Figure 8) indicated that fresh chicken samples generally had higher fecal coliform counts compared to frozen samples. In particular, samples from Zone A showed the highest contamination levels, followed by Zones B, C, D, E, and F in descending order. Fecal coliforms, particularly those originating from improper sanitation practices, are a key indicator of meat contamination. High fecal coliform counts are associated with poor hygienic conditions during processing and handling [23, 29].
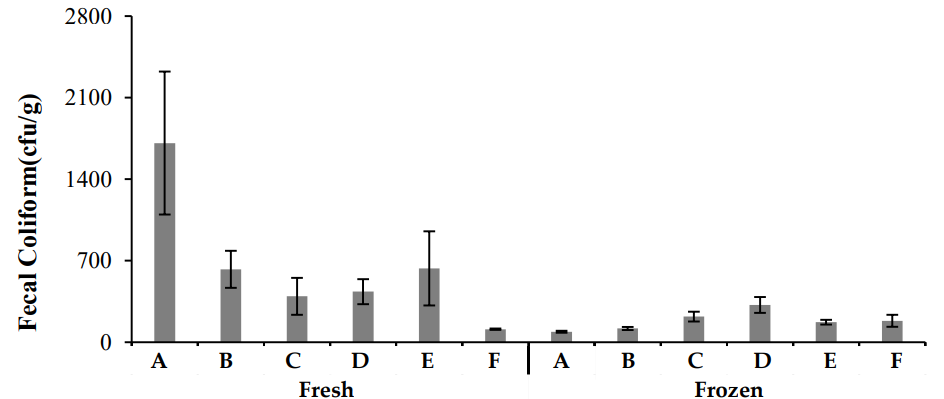
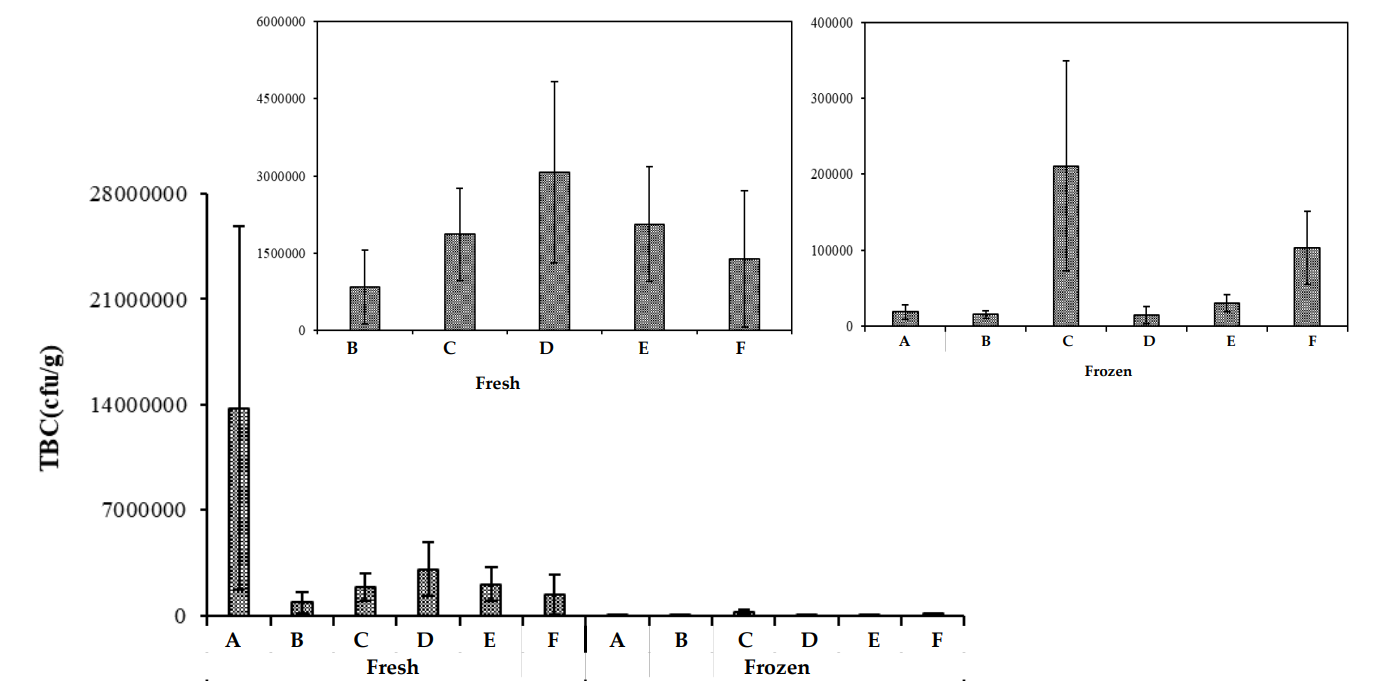
3.3.5 Total Bacterial Count
Total bacterial count (TBC) is a standard method for assessing the microbiological quality of meat [30]. Chicken meat, both fresh and frozen, is prone to bacterial contamination, which can lead to foodborne illnesses [31]. The TBC results for fresh and frozen chicken samples from different zones are presented in Figure 9. The mean TBC values for frozen chicken ranged from 1.38x10⁷ to 1.39x10⁶, while the mean TBC for fresh chicken ranged from 1.83x10⁴ to 2.11x10⁵. These findings indicate that frozen chicken meat had higher bacterial loads compared to fresh meat, in line with other studies [32, 33].
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bhandari, N.; Nepali, D. B.; Paudyal, S. Assessment of Bacterial Load in Broiler Chicken Meat from the Retail Meat Shops in Chitwan, Nepal. Int. J. Infect. Microbiol. 2013, 2 (3), 99–104. [Google Scholar] [CrossRef]
- Hameed, T.; Asmat, T. M.; Tariq, M. M.; Bajwa, M. A.; Rafeeq, M.; Hilal, B.; Attique, M. A.; Bokhari, F. A. Study on Current Status and Future Trends of Commercial Poultry Production in Pakistan. Pure Appl. Biol. 2017, 106 (1), 190–196. [Google Scholar] [CrossRef]
- Magsi, H.; Randhawa, A. A.; Shah, A. H. Halal Meat Production in Pakistan: Status and Prospects. J. Islamic Mark. 2021, 12 (5), 941–950. [Google Scholar] [CrossRef]
- Arthur, T. M.; Bosilevac, J. M.; Brichta-Harhay, D. M.; Kalchayanand, N.; Shackelford, S. D.; Wheeler, T. L.; Koohmaraie, M. Effects of a Minimal Hide Wash Cabinet on the Levels and Prevalence of Escherichia coli O157:H7 and Salmonella on the Hides of Beef Cattle at Slaughter. J. Food Prot. 2007, 70 (5), 1076–1079. [Google Scholar] [CrossRef]
- Leeson, S. Vitamin Requirements: Is There Basis for Re-Evaluating Dietary Specifications? World’s Poult. Sci. J. 2007, 63 (2), 255–266. [Google Scholar] [CrossRef]
- Park, S.; Worobo, R. W.; Durst, R. A. Escherichia coli O157:H7 as an Emerging Foodborne Pathogen: A Literature Review. Crit. Rev. Food Sci. Nutr. 1999, 39 (6), 481–502. [Google Scholar] [CrossRef]
- Easa, S. M. The Microbial Quality of Fast Food and Traditional Fast Food. Nat. Sci. 2010, 8 (10). [Google Scholar]
- Soofi, S. B.; Habib, M. A.; von Seidlein, L.; Khan, M. J.; Muhammad, S.; Bhutto, N.; Khan, M. I.; Rasool, S.; Zafar, A.; Clemens, J. D.; Nizami, Q. A Comparison of Disease Caused by Shigella and Campylobacter Species: 24 Months Community-Based Surveillance in 4 Slums of Karachi, Pakistan. J. Infect. Public Health 2011, 4 (7), 12–21. [Google Scholar] [CrossRef]
- Okoh, P. I.; Okoruwa, M. I.; Okosun, S. E. A Comparative Assessment of the Microbial Load of Beef and Chicken Meat Collected at Different Hours of the Day in Ekpoma Town Market. Eur. J. Nutr. Food Saf. 2019, 31, 365–371. [Google Scholar] [CrossRef]
- Le-Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and Food Poisoning. Genet. Mol. Res. 2003, 2 (1), 63–76. [Google Scholar]
- Rodriguez-Turienzo, L.; Cobos, A.; Moreno, V.; Caride, A.; Vieites, J. M.; Diaz, O. Whey Protein-Based Coatings on Frozen Atlantic Salmon (Salmo salar): Influence of the Plasticiser and the Moment of Coating on Quality Preservation. Food Chem. 2011, 128 (1), 187–194. [Google Scholar] [CrossRef]
- Ciobanu, M. M.; Lazar, R.; Boisteanu, P. C. Influence of Temperature and Freezing Time on Broiler Chicken Meat Colour. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2015, 73 (1), 55–58. [Google Scholar]
- Jett, B. D.; Hatter, K. L.; Huycke, M. M.; Gilmore, M. S. Simplified Agar Plate Method for Quantifying Viable Bacteria. Bio-techniques 1997, 23, 648–650. [Google Scholar] [CrossRef]
- Rahman, U.; Sahar, A.; Pasha, I.; Rahman, S. U.; Ishaq, A.; Sohaib, M.; Chughtai, M. F. J.; Zafar, H. Augmenting Quality and Microbial Safety of Broiler Meat at Refrigeration Storage by Applying Chemical Interventions. J. Food Process. Preserv. 2017, 41, e13030. [Google Scholar] [CrossRef]
- Kamat, A. S.; Alur, M. D.; Nerkar, D. P.; Nair, P. M. Hygienization of Indian Chicken Meat by Ionizing Radiation. J. Food Saf. 1991, 12 (1), 59–71. [Google Scholar] [CrossRef]
- Basar, M. A.; Rahman, S. R. Assessment of Microbiological Quality of Processed Fruit Juice. Bangladesh J. Microbiol. 2007, 24 (2), 166. [Google Scholar]
- Hernandez, T.; Sierra, A.; Rodriguez-Alvarez, C.; Torres, A.; Arevalo, M. P.; Calvo, M.; Arias, A. Salmonella enterica Serotypes Isolated from Imported Frozen Chicken Meat in the Canary Islands. J. Food Prot. 2005, 68 (12), 2702–2706. [Google Scholar] [CrossRef]
- Pini, P. N.; Gilbert, R. J. The Occurrence in the UK of Listeria Species in Raw Chickens and Soft Cheeses. Int. J. Food Microbiol. 1988, 6 (4), 317–326. [Google Scholar] [CrossRef]
- Van Nierop, W.; Duse, A. G.; Marais, E.; Aithma, N.; Thothobolo, N.; Kassel, M.; Stewart, R.; Potgieter, A.; Fernandes, B.; Galpin, J. S.; Bloomfield, S. F. Contamination of Chicken Carcasses in Gauteng, South Africa, by Salmonella, Listeria monocytogenes, and Campylobacter. Int. J. Food Microbiol. 2005, 99 (1), 1–6. [Google Scholar] [CrossRef]
- Chan, J. T.; Omana, D. A.; Betti, M. Functional and Rheological Properties of Proteins in Frozen Turkey Breast Meat with Different Ultimate pH. Poult. Sci. 2011, 90 (5), 1112–1123. [Google Scholar] [CrossRef]
- Jia, B.; Yoon, S. C.; Zhuang, H.; Wang, W.; Li, C. Prediction of pH of Fresh Chicken Breast Fillets by VNIR Hyperspectral Imaging. J. Food Eng. 2017, 208, 57–65. [Google Scholar] [CrossRef]
- Hashim, I. B.; Resurreccion, A. V.; McWalters, K. H. Descriptive Sensory Analysis of Irradiated Frozen or Refrigerated Chicken. J. Food Sci. 1995, 60 (4), 664–666. [Google Scholar] [CrossRef]
- Odwar, J. A.; Kikuvi, G.; Kariuki, J. N.; Kariuki, S. A Cross-Sectional Study on the Microbiological Quality and Safety of Raw Chicken Meats Sold in Nairobi, Kenya. BMC Res. Notes 2014, 7 (1), 627. [Google Scholar] [CrossRef]
- Jaja, B. N.; Saposnik, G.; Lingsma, H. F.; Macdonald, E.; Thorpe, K. E.; Mamdani, M.; Steyerberg, E. W.; Molyneux, A.; de Oliveira Manoel, A. L.; Schatlo, B.; Hanggi, D. Development and Validation of Outcome Prediction Models for Aneurysmal Subarachnoid Haemorrhage: The SAHIT Multinational Cohort Study. Stroke 2018, 49 (2), 360–368. [Google Scholar] [CrossRef]
- Mannino, D. M.; Homa, D. M.; Akinbami, L. J.; Moorman, J. E.; Gwynn, C.; Redd, S. C. Surveillance for Asthma—United States, 1980–1999. MMWR Surveill. Summ. 2002, 51 (1), 1–3. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Escherichia coli O157:H7 Infections Associated with Eating Ground Beef—United States, June–July 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51 (29), 637–639. [Google Scholar]
- Scallan, E.; Hoekstra, R. M.; Angulo, F. J.; Tauxe, R. V.; Widdowson, M. A.; Roy, S. L.; Jones, J. L.; Griffin, P. M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17 (1), 7. [Google Scholar] [CrossRef]
- Hulebak, K. L.; Schlosser, W. Hazard Analysis and Critical Control Point (HACCP) History and Conceptual Overview. Risk Anal. 2002, 22 (3), 547–552. [Google Scholar] [CrossRef]
- Mataragas, M.; Skandamis, P. N.; Drosinos, E. H. Risk Profiles of Pork and Poultry Meat and Risk Ratings of Various Pathogen/Product Combinations. Int. J. Food Microbiol. 2014, 126 (1–2), 1–12. [Google Scholar] [CrossRef]
- Khalafalla, F. A.; Ali, H. M.; El-Fouley, A. Microbiological Evaluation of Chicken Meat Products. J. Vet. Med. Res. 2019, 26 (2), 151–163. [Google Scholar] [CrossRef]
- Wahab, A. H.; Ibraheem, I. A. Assessment of Microbial Effort of Some Types of Frozen Poultry in Markets Beyond Local City of Al-Hilla/Babylon Governorate. Al-Kufa Univ. J. Biol. 2016, 45–49. [Google Scholar]
- Al-Dughaym, A. M.; Altabari, G. F. Safety and Quality of Some Chicken Meat Products in Al-Ahsa Markets-Saudi Arabia. Saudi J. Biol. Sci. 2010, 17 (1), 37–42. [Google Scholar] [CrossRef]
- Ibrahim, H. M.; Amin, R. A.; Ibrahem, I. A.; Yunis, O. F. Isolation of Enterobacteriaceae from Poultry Products in El-Behera and Alexandria Governorates. BVMJ 2014, 27 (1), 109–117. [Google Scholar]
Copyright: © 2024 by the authors.
License: This article is published under the Creative Commons Attribution 4.0 International.CC BY 4.0
Publisher: Insights Academic Publishing (IAP), Lahore, Pakistan.

