Beyond Antibiotics: A Review of Sustainable Strategies and Emerging Alternatives for Poultry Health Management in Modern Farming
by
Abdul Mateen 1,
Muhammad Arslan 1,
Rabia F. Ali 2,
Muhammad Usman 3 and
Usman Elahi 4, *
1
School of Agricultural Technology and Food Industry, Walailak University, Nakhon Si Thammarat 80160, Thailand
2
Lahore College for Women University, Lahore 54000, Pakistan
3
Department of Poultry Production, Faculty of Animal Production and Technology, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan
4
Faculty of Agriculture and Veterinary Sciences, Superior University, Lahore 54000, Pakistan
*
Correspondence: usmanelahi@gmx.de
Insights Anim. Sci. 2025, 2(1), 1–22.
https://doi.org/10.69917/ias.02.01-01
Received: January 22, 2025 /
Accepted: April 10, 2025 /
Published online: May 14, 2025
Abstract
Antimicrobial resistance (AMR) has made it difficult for both people and animals to control disease using antibiotics, which has led to food insecurity, particularly for the chicken business. As a result, it is necessary to create long-term plans for keeping chicken flocks healthy as well as possible, and side-effect-free antibiotic substitutes. The use of probiotics and prebiotics, vaccination and immunostimulants, organic farming, improved hygiene and biosecurity measures, in-ovo-inoculation, feed additives, and nanoparticles are some of the practices that are currently being used to minimise the use of antibiotics and to maintain the optimal health, immunity, gut integrity, and growth performance of birds. However, because of their potential use in replacing antibiotics in poultry, certain new alternatives—such as antimicrobial peptides, bacteriophages, enzymes and enzyme-based products, and nanoparticles—are receiving a lot of attention. To preserve chicken health and reduce the need for antibiotics, the study emphasizes the potential of these sustainable practices and new alternatives. This will ultimately aid in the fight against AMR and guarantee the production of safe and reasonably priced poultry protein.
Keywords:
antimicrobial resistance; poultry health; poultry production; antibiotics alternatives
1. Introduction
The current population of the world is to be about 8.1 billion [1] and will reach 9.8 billion by 2030, with a huge increment in population in developing countries of Asia and Africa [2]. Due to this increase in population, overall food demand will surge by more than 50 % and food from animal sources by approximately 70% [3]. World Health Organization (WHO) recommended that in our daily basic diet, there should be usage of different types of foods like maize, wheat, rice, and potatoes including leg-umes like lentils and beans, along with ample quantity of fresh fruit and vegetables and food from animal sources (e.g., fish, meat, egg and milk)[4]. To deal with increas-ing insecurity of food (protein), sub-therapeutic administration of antibiotics in poul-try farms is a routine practice to compensate for the overcrowding and the unhygienic environments [5].
Antibiotics are being used as a growth promoter and for the prevention and treatment of diseases in the poultry sector of many developing countries. Their usage for growth enhancement often requires a very small amount of administration as compared to therapeutic use and this also develops resistance to antibiotics in bacteria. The occurrence of antibiotic resistance in bacteria and horizontal transfer of antibiotic resistance genes result in a compromise in nutritional and the economic prospects of poultry and other livestock animals that are used for food production [6].
A study demonstrated the usage pattern of antibiotics in selected farms in Bang-ladesh and reported the use for therapy is 43.8%, for prevention is 31.5%, and for both 47.9% and 8.2% for growth promotion [7]. However, the usage of antibiotics from na-tion to nation depends upon several factors, i.e. related knowledge, the economy of the country, management system, and the routine practices of farming. A study in 2015 revealed that on average, global annual usage of antimicrobials/kg of chicken meat production was 148 mg and is estimated to increase by 67% between 2010 and 2030 [8]. From 2000 to 2010, antibiotic usage in 71 countries increased by 36% although Brazil, Russia, India, China, and South Africa (BRICS) contributed to about 75% of this huge increment [9]. This widespread and irrational use of antibiotics is encouraging antimicrobial resistance in living organisms. This AMR is a very serious global health issue for humans and animals equally affecting developed and developing nations [5] reducing the capability for treatment of the infections caused by bacteria and also creating threats associated with morbidity and mortality by these antibiotic-resistant bacteria [9]. There should be an investigation of alterna-tive approaches to replace antibiotics to maintain gut health and ensure the produc-tion of good quality and cheap poultry protein [8].
This study aims to review the sustainable strategies (organic farming practices, In ovo inoculation, improved hygiene and biosecurity measures, vaccination and im-munostimulants) and alternatives to antibiotics (bacteriophages, antimicrobial pep-tides, feed additives, enzymes and enzyme-based products, probiotics and prebiotics, nanoparticles) in poultry production to cope with the issue of increasing antibiotic re-sistance. This review will highlight the usage of antibiotic alternatives and how it is beneficial for poultry and will also suggest potential alternatives.
2. Sustainable Strategies in Poultry Health Management
There are many sustainable strategies that we can utilize for the maintenance of our poultry flock’s health. With good management and the implication of these strate-gies, we can reduce antibiotic usage and make our birds healthier. These are as follows:
2.1. Organic Farming Practices
Organic animal farming should adhere to strict animal welfare guidelines and improve the health and wellbeing of the animals, paying particular attention to the behavioral needs of different species [10]. European Union regulation on the production of organic broilers demands low stocking densities (33 kg/m2 ) with outdoor access to birds [11] so to provide them an opportunity to perform their natural behaviors [12]. Natural light is very crucial and when managed with artificial light should be given at least eight hours [10]. To provide the best possible health under these conditions, we need to select the most suitable hybrid birds in this regard [13]. Therefore, the fast-growing broilers are being replaced by the slow-growing hybrids as they are better adapted to organic production conditions and welfare concerns [10]. Fast-growing broilers also showed severe complications in movement because of the high growth rate and ultimately body weight that results in keeping the bird more at the resting stage, especially in the last week of production (rearing). The reduction in movement causes leg weakness and results in skin lesions and blisters due to long resting periods. However, slow-growing genotypes are more active and with increased usage of perches and showed better adaptability [14]. Thus, organic farming in poultry production en-sures more health and greater welfare coverage and thus should be used as a suitable strategy than intensive farming.
Seven key features are considered to have a role in improving the welfare of poultry in organic farming: suitable breeds, no mutilations, access to outdoor environment, availability of natural light, perch space and elevated sitting levels, roughage provision and reduced stocking density. The minimum requirements as established by the EU regulations on the production of poultry birds are presented in Table 1 to highlight some of the specific features intended to improve animal welfare in the production of poultry. In addition to the minimal guidelines ensuring the safety of laying hens and broiler chickens in conventional farming [15] to maintain equilibrium between the welfare of birds, adaptability to the environment, biodiversity and productive performance [14].
Table 1. European Union minimum criteria for the housing and management of laying hens (Directive 1999/74/EC) and broiler chicks (Directive 2007/43/EC) in both conventional and organic poultry farming (EU regulations 2018/848 and 2020/464).
| Parameters | Laying hens | Broilers | |||
|---|---|---|---|---|---|
| Conventional | Organic | Conventional | Organic | ||
| Stocking density | Usable indoor area: 9 hens/m² | Usable indoor area: 6 hens/m² | 33 kg/m² | 21 kg/m² | |
| Perches and/or raised sitting areas | 15 cm perch/hen | 18 cm perch/hen | Not required | Each chicken has a 5 cm perch and/or a 25 cm² elevated sitting level. | |
| Outdoor access | Not required | 4 m²/chicken and one-third of life | Not required | 4 m²/chicken and one-third of life | |
| Lighting | Enough light to see each other, explore the environment, and exhibit typical levels of activity | Natural light | Lighting at least 80% of the space with a maximum of 20 lux | Natural light | |
| Nocturnal rest (hours per day with no artificial light) | About one-third of the day | Continuous 8 h | 4 h of the 6 h are continuous. | Continuous 8 h | |
| Mutilations (beak trimming) | Permitted to avoid cannibalism and feather pecking (<10 days old) | Only as an exception (≤3 days old), otherwise, it is not permitted | Permitted to avoid cannibalism and feather pecking (<10 days old) | Only as an exception (≤3 days old), otherwise, it is not permitted | |
| Roughage | No requirement | Constant availability of adequate amounts when kept indoors | No requirement | Constant availability of adequate amounts when kept indoors | |
| Growth rate | N/A | N/A | Fast-growing breeds permitted | Slow-growing breeds or those raised for at least 81 days | |
2.2. In Ovo Inoculation
When eggs are transferred from setters to hatchers, which typically occurs between 17.50 and 19.20 days of incubation, several inoculation procedures are employed in the hatchery to improve the health of the chicks [16]. Vaccines have been approved for Marek’s disease (MD), infectious bursal disease virus (IBDV), fowl pox, Newcastle, and coccidiosis for the in ovo delivery [16, 17]. The efficacious protection against Marek's disease was observed when vaccine was administered in the body of embryo or the amnion. Several vaccines, medications, hormones, competitive exclusion cultures, and other supplemental nutrients have been continuously studied in labs to improve im-munity and growth. The results of these studies have also been published, but they are not yet commercially available [18]. By using the in ovo technique, numerous nutrients, such as egg white, amino acids, peptides, carbohydrates, nucleotides, electrolytes, vitamins, L-carnitine, creatinine, and plant extracts have also been tested for their ability to improve embryonic development, hatchability, and post-hatch performance [19]. Similarly, it has been noted that in ovo nutrient delivery at the late-term embryonic stage may reduce hatchling mortality during crucial post-hatch times [20] and ultimately prove to be an impactful strategy in poultry health management.
2.3. Improved Hygiene and Biosecurity Measures
According to the Food and Agriculture Organization (FAO), biosecurity is defined as offering a strategic and unified approach to evaluate and manage the risks in food safety, health, life, and biosafety of animals and plants [21]. From the last decade, the importance of implementation of strict biosecurity has increased significantly with globalization, intensification in livestock farming systems, and consequent increasing food trade and international, which also cause the emergence and re-emergence of diseases [22]. Meat production through broiler farming without the usage of antibiotics demands reduction in stocking density, increment in the downtime, rapid and frequent cleaning with strict biosecurity measures. It is very easy for microorganisms to take entry into the farms and thus affect the production performance of the poultry flocks, so, biosecurity must be implemented without leaving any gap [8]. Biosecurity is not only for infectious diseases but also covers the other possible hazards, and for instance, residues, pests, and it also covers the antimicrobial usage and antimicrobial resistance [22]. There should be proper consideration of hygiene at poultry farms and some critical hygiene steps with very limited implementation by the farmers are the cleaning and disinfection of the feed storage silo, disinfection of the houses and the waterlines in the houses, specific areas to enter in poultry houses for collection of eggs, different labor and staff between poultry sheds and the egg storage room, lack of hand washing before getting entrance into the house and proper disposal of the mortality [23]. Antimicrobial usage is closely linked to suboptimal conditions of animal husbandry including poor hygiene and biosecurity. We can reduce antibiotic usage by adopting the intervention that fo-cuses on infrastructure improvement, farming, and cleaning conditions [24]. Therefore, by implementation of all these hygiene measures under strict biosecurity will ensure the flock's health with minimal bacterial load and thus no antibiotic usage is required.
2.4. Vaccination and Immunostimulants
One of the main causes that results in huge economic losses in the poultry industry is viral outbreak and it negatively affects the zootechnical performances of birds, like feed intake, weight gain, Feed Conversion Ratio (FCR), egg quality, and meat production therefore, preventive measures should be taken including vaccination. Live and killed vaccines have a long usage history on poultry farms as a prophylactic against viral diseases with economic and global significance [25]. Vaccination programs play crucial role in controlling diseases and provide a cost-effective way to lessen the bacterial and viral load. Bacterial vaccines can reduce the usage of antibiotics in the poultry industry. Bacterial vaccines perform their action by activating the immune system of poultry birds in response to specific bacterial pathogens and thus provide long-term protection from future infections. Ultimately helpful in reducing antimicrobial resistance (AMR) [26]. Shimma et al., [27] found that inactivated bacterin made from chicken embryos infected with fowl cholera is effective against infections caused by either homologous or heter-ologous serotypes of Pasteurella multocida.
Immunostimulants are compounds that have immunotropic properties and can maintain the normal state of the immune system. Plant-based immunostimulants have got significant due to immunostimulating effects, and the stimulation of endocrine and nervous systems [28]. A study with oral administration of 200 mg/kg extract of Plec-tranthus parviflorus before vaccination to poultry flock resulted in a significant positive effect on ND and IBD humoral response [29]. Another study regarding the usage of immunostimulant antiviral medicinal plant i.e., neem, resulted in complete protection of chicken flocks against the highly pathogenic avian influenza (HPAI) (H5N8) and prevented the shedding of the virus [30]. The lecithin extracted from mushrooms in-creased the antibodies titers against IBD as compared to the commercial lecithin [31]. Thus, by the utilization of vaccination and immunostimulants, we can protect our flock from diseases and maintain the health of our birds.
3. Emerging Alternatives to Antibiotics
With a continuous increase in modern farming, the prevention and treatment of diseases especially caused by bacteria in poultry farming is a huge problem and needs to be solved. Historically, antibiotics have been used to stop the amplification of gastrointestinal pathogens, to promote the animal’s growth and to improve the FCR. However, this long-term usage of antibiotics has created resistance in pathogens against antibiotics and that’s why there is an urgent need to replace antibiotics usage with less expensive and more efficient alternatives to ensure the sustainable development of the poultry industry [32]. Some alternatives have been shown in Figure 1.
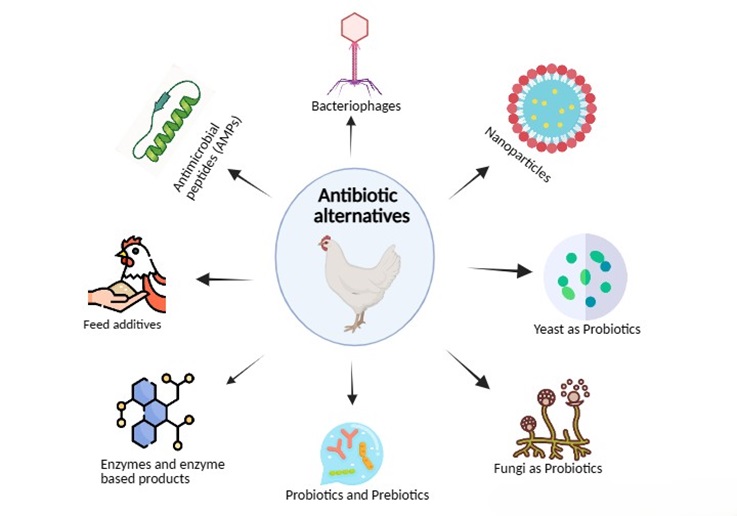
Figure 1. Emerging antibiotics alternatives.
3.1. Bacteriophages
Bacteriophages are a group of viruses that target certain specified bacteria and have less distribution in the environmental niches like hydrothermal vents of the ocean and sediments but are also found in surface life forms in the kingdom Animalia and Plantae [32]. European Medicines Agency approved the first scientific guideline on bacteriophage-based veterinary medicines for livestock and poultry production activities, and the guidelines define this phage-based therapy as a novel technology with a great focus on safety and residues of this therapy, also imposing specific requirements for animal clinical trials [33]. Practically, bacteriophages are administered in the bacteriophage cocktails form that contains a mixture of multiple bacteriophages at various time intervals, to prevent the development of resistance to a specific bacterio-phage type by bacteria. To achieve maximum effectiveness, it entails the invasion and lysis mechanism of many bacteriophages that target the same bacterial hosts and related microorganisms [32]. Supplementation of 400 mg/kg of bacteriophage cocktail in the diet of weaned piglet that targets Salmonella, Escherichia coli, Staphylococcus aureus, and Clostridium perfringens and resulted in a significant increase in daily feed intake on an average, improved the integrity of intestine, inhibited the intestinal inflammation and reduced the occurrence of diarrhea in piglet [34]. A study with the addition of 1.5 g per kilogram of bacteriophage cocktail in the diet of weaned piglets resulted in a significant increase in ileal Lactobacillus spp. and a lower the level of E.coli and Clostridium challenge in ileum to about 5.76% and 4.21% respectively and also showed significantly better average daily gain [35]. They are in more focus as antibiotic alternatives due to their diverse nature with greater selectivity, operability, more precise targeting ability, rapid absorption, and killing of bacteria without disrupting the normal body bacteria balance.
Despite all the advantages, bacteriophages have some limitations like narrow specificity, which limits their effectiveness against some bacterial strains until the complex cocktails are fully formed [36]. Bacterial resistance against bacteriophages also develops with time, and posing safety concerns and thus is considered critically.
3.2. Antimicrobial Peptides (AMPs)
AMPs are a class of small peptides and have the potential to stimulate the innate immune system against pathogens and can also stimulate defensive mechanisms against infectious and non-infectious pathogenic agents like bacteria, parasites, fungi, and viruses. AMPs have a role in enhancing immunity and can be used as an alternative to antibiotics [37]. They are potent multifunctional therapeutic agents, active against many microorganisms, and are called natural antibiotics. Some AMPs result in the death of Gram-positive and Gram-negative bacteria within a few minutes and are considered an ideal candidate for pharmacological applications. They affect microorganisms either by membrane targeting which results in impairment of the cell membrane structure or by inhibiting nucleic acid synthesis, essential enzymes, and functional proteins [38]. In a study with administration of AMP HJH-3 with concentration 100 μg/ml after co-incubation for 6 h resulted in good antimicrobial activity, low hemolysis, and with no cytotoxicity and could effectively kill S. Pullorum [39]. In another study, the supplementation of AMP (Cecropin A) vs antibiotics in the diet of zebrafish was done to observe the effects of the intervention on the microbiota. The findings suggested that antibiotic usage resulted in the occurrence of multidrug-resistant bacteria and Cecropin A did not take part in this phenomenon [40]. Microcin C7 administration in the diet of broilers with a dosage 6 mg/kg resulted in reduced chemokine TNF-α and IL-8, increased serum cytokine IL-10 in serum [41]. In another study with microcin J25 in broiler chicken at dosage 0.5 and 1mg/kg MccJ25 resulted in downregulation of proinflammatory cytokines, TNF-α, IL-1β, and IL-6 in the serum of E. coli and Salmonella challenged broiler birds [42]. Piscidin usage in yellow feather chicken resulted in deduced IL-17A, IL− 22, IFN-α, IFN-у, IL-1β, IL6 and improved the average daily gain at 100 and 200 mg/k [43]. Antimicrobial peptide sublancin invitro study with minimum inhibitory concentration (MIC) (8 µM) against C. perfringens in comparison to lincomycin resulted in reduced e IL− 1β, IL− 6, TNF-α levels (p < 0.01) in comparison to lincomycin, reduced the necrotic enteritis induced by clostridium perfringens and also exhibited the sample result in broiler for 28-d period [44]. ε-polylysine hydrochloride (ε-PLH) resulted in improved production in laying hens by reducing the abundance of Desulfovibrio and Streptococcus in cecum microbiota [45]. Histone H5 derived from chicken erythrocytes with MIC (4.9 µg.mL/1 against E. coli; 1.9 µg.mL/1 against Salmonella Typhimurium) showed potential to be a novel antimicrobial peptide [46]. AMPs are excellent antibiotic alternatives with good antimicrobial and immunomodulatory potential, but their high cost of production and less stability in the gastrointestinal tract (GIT) hinder their wider application. There are also toxicity risks to the host cells, and further research is needed to understand them completely [47].
3.3. Feed Additives
Due to the ban on antibiotics by the European Union in 2006, more interest has been shifted toward the usage of feed additives as an alternative to antibiotics, and many feed additives are being utilized in the poultry sector. Different feed additives from herbal extracts, essential oils, and organic acids have been widely used for the replacement of AGPs [48]. Prebiotics and probiotics are also in the priority lists and widely used as feed additives but are discussed later in another section. Phytogenic is a broad category of feed additives in poultry and has effects on the improvement of gut health and other physiological functions. Distinctive examples are rosemary derivatives, thyme, oregano, sage, cinnamon, citrus, pepper, and anise, however, the phytogenic efficacy depends on several factors, like composition, level of inclusion in feed, feed composition, and bird’s genetics [48]. The inclusion of different feed additives benefits the birds in several ways. The addition of herbal compounds results in the improvement of the immune system, antioxidant status and stimulates the activity of digestive enzymes and has good efficacy in controlling pathogenic bacteria [49]. Essential oil mixture with garlic and lemon resulted in improved average body weight gain, feed conversion ratio (FCR), and enhanced intestinal microbial content [50]. Minerals and vitamins have also been proved to be a potent source for the improvement of the health of birds. Vitamin C, is a water-soluble nutrient having role as powerful antioxidant, protecting cells from damage caused by free radicals, supports immune system, and also helpful in wound healing [51, 52]. Vitamin C improved the performance status and enhanced the immunological traits of birds [53]. The addition of zinc, selenium, and magnesium improved the immunity and the antioxidant status of birds [54]. Organic acids also play an important role and are used as feed additives [55]. Organic acids (OC) are compounds that are organic in nature and have weak acidic properties, classified according to the number of carboxylic acid groups (R-COOH). They are considered as a promising alternative to antibiotics as they have antimicrobial effects in animal feed [54]. Citric acid usage resulted in improved growth performance, reduction in abdominal fat, ileal E. Coli and Coliform [55]. Formic acid usage resulted in increased body weight gain and decreased pH and coliform counts [56] and butyric acid improved the body weight (BW) gain and FCR [54]. Two organic acids showed synergistic effect with organic acid 1 (OA1) constituted of butyrate, medium-chain fatty acids, organic acids, and phenolics; while organic acid 2 (OA2) based on buffered short-chain fatty acids protected the broiler chickens with Clostridium perfringens (CP) type A challenge from severe intestinal lesions, oxidative stress [57]. Utilization of Mentha arvensis and Geranium thunbergii in drinking water from 0.01% to 0.1% resulted in a significant increment in the egg production, reduced the ammonia production from excreta and increased the immunity of laying hens by increasing the serum interleukin-6, tumor necrosis factor α, and immunoglobulins (IgA and IgG) [58]. Essential oil of Citrullus lanatus, when used at up to 2 g/ kg of feed, resulted in improvement in the egg production and strength of the tibia bone [59]. Due to these reasons, we can employ feed additives as a potent and long-term substitute for antibiotics, thus increasing future productivity and poultry health.
There is also a concern that feed additives, especially phytogenic feed additives, show inconsistent results in the growth performance and pathogen inhibition due to the variability in the composition of bioactive compounds in plants [60]. The lack of standardization in the formulations and their overuse can damage the digestive system's efficiency [60].
Table 2. Antibiotic alternatives, their mode of action, effectiveness, cost consideration, and challenges in application.
| Alternative | Description | Mechanism of action | Effectiveness | Cost consideration | Implementation Challenges | Sources |
|---|---|---|---|---|---|---|
| Bacteriophages | Viruses that target specific bacteria and replicate inside them. | Shows high effectiveness against specific strains and has minimal effect on the gut microbiota and performs effective killing of bacteria without disrupting the normal body bacteria balance. Helpful in reducing the Salmonella, E. coli and Campylobacter | Shows high effectiveness against specific strains and has minimal effect on the gut microbiota and performs effective killing of bacteria without disrupting the normal body bacteria balance. Helpful in reducing Salmonella, E. coli and CampylobacterExample:ST4, L13, and SG3 bacteriophages at108 PFU/kg in layers showed decreased mortality and bacterial re-isolation from the organs | Moderate to high cost and varies and depends upon the target strain. | Highly specific to their bacterial hosts, which can limit their broad application across different bacterial strains.The bacterial resistance issue also resides. | [32, 61-64] |
| Antimicrobial peptides | Small molecules that range in mass from 1 to 5 kDa. They work by interacting with the target species' cell membranes. | Causes impairment of the bacterial membrane, inhibits the synthesis of nucleic acid, essential enzymes, functional proteins and enhances the immune response, acts as an anti-inflammatory agent | Broad-spectrum activity, effective against gram-positive and gram-negative bacteria, rapid action, low resistance development, enhanced immune response. Example: Antimicrobial peptide-A3 at 60 and 90 mg/kg diet in broilers had improved average daily feed intake (ADFI) and average daily gain (ADG) | High (high cost of synthesis and purification of AMPs) | Bacterial resistance issue. Utilization in feed, especially the stability aspect, large-scale production, and AMPs degradation in the gastrointestinal tract. Preparation methods are costly. | [47, 64-69] |
| Feed additives | Products that enhance the quality of feed and food from animal origin also enhance the animals’ performance and health | Perform action by modulating the gut microbiota, reducing the colonization of pathogens and enhance nutrient absorption | Showed moderate effectiveness in reducing harmful bacteria. Also present anti-inflammatory and antioxidant activity. Improved growth efficiency/egg production, prevent disease, and improve feed utilization. Example: The usage of Garlic at 1% of the diet in broilers resulted in improved growth performance, carcass quality, nutrient digestibility, and lipid profile | Low to moderate cost based on the ingredient purity and processing | Stability and administration issues. Delivery to the target site due to degradation. Variation in efficacy across different environments and high doses may alter gut microbiota negatively. | [70-73] |
| Enzymes and enzyme-based products | Proteins that catalyze a chemical reaction but are not altered themselves | Breakdown antinutritional factors like NSPs, Phytates, etc. | Improved apparent metabolizable energy (AME), improved digestion and absorption of nutrients, particularly fat and protein, and decreased digesta viscosity. Example: The usage of α-amylase, β-glucanase at 400 g ton−1, and xylanase at 50g ton−1 in broilers diet showed improvement in the digestion and absorption of the nutrientswith reduction in the viscosity of digesta | Moderate cost based on the enzyme type, stability and dosage | Require optimal conditions, interaction with other feed components like vitamins, minerals, and other additives. | [74-77] |
| Prebiotics | Prebiotics are non-digestible food ingredients that stimulate the growth of beneficial bacteria in the gut | while prebiotics serve as food for probiotics, boosting their growth and activity | Improved health and production performance. Prebiotics in broilers diet, like Mannan oligosaccharides (MOS) at 0, 0.5, 1, and 1.5g/kg, showed increased weight of bursa and jejunal immunoglobulin M (IgM), and immunoglobulin G (IgG) content. | Low to moderate | Handling of prebiotics and proper dose standardization. | [78, 79] |
| Probiotics | Probiotics are microbials that can be described as living, beneficial microbial feed supplements | Probiotics compete with pathogens for obtaining nutrients and attach at the site of the gut, thus reducing the colonization of harmful bacteria | Improved feeding efficiency, gut health, immune response and performance. Effective against pathogens like Clostridium perfringens, Salmonella spp. Example: Probiotics supplementation in the laying hen diet 0.5 g/kg Clostridium butyricum, showed improved average daily feed intake (AFDI), an increase in FCR, eggshell strength% in albumen and a decrease in reactive oxygen species (ROS) level in ileum and cecum and reduction in malondialdehyde (MDA) in the serum. Prebiotics in broilers diet, like Mannan oligosaccharides (MOS) at 0, 0.5, 1, and 1.5g/kg, showed increased weight of bursa and jejunal immunoglobulin M (IgM), and immunoglobulin G (IgG) content. | Low to moderate based on the selection of strain and formulation | Probiotics viability in feed and storage; survival through the GIT. Difficulty in oral administration | [80-82] |
| Nanoparticles | Tiny particles from 1-100 nm in size offer unique properties because of their smaller size | Disruption of the bacterial cell membrane generates reactive oxygen species (ROS), interferes with bacterial DNA and protein synthesis | Highly effective against multiple pathogens, including antibiotic-resistant bacteria. Having a role in enhancing immunity and gut function. Example:Nanoparticles of zinc oxide in laying hens resulted in an improvement in FCR, hen day egg production, egg mass, Haugh units, egg shell thickness and egg shell percentage. Decrement in serum cholesterol, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, urea and somewhat creatinine | Having high production costs | Toxicity concerns, need careful dose optimization | [83-85] |
3.4. Enzymes and Enzyme-based Products
Enzymes are proteins that can accelerate a specific chemical reaction. Enzymes in feed can be divided into two types: endogenous and exogenous depending on the substrate. Endogenous enzymes are produced by the animal body and play a role in the breakdown of basic feed components like fat, protein, and carbohydrates [48]. However, exogenous enzymes can be added externally in the feed that can remove the cell wall of raw material, degrade the non-starch polysaccharides (NSP), minimize the chyme velocity, boost the nutrient intake, reduce the antinutritive activity and in this way, enhance the animal growth performance [86]. Thus, the inclusion of enzymes in feed acts as growth promoters and decomposes large molecules into prebiotics [87]. The addition of β-mananase in the diet of broiler chicken hydrolyzed β-manans, reduced the intestinal content viscosity, enhanced the nutrient digestibility, and improved the environment of the intestine [88]. Results of a study suggested that the addition of Bacillus-DFM (direct fed microbials) in poultry diet, releases a variable set of enzymes and other antimicrobial compounds that result in performance enhancement by improving nutrient digestibility, lowering the intestinal viscosity, maintenance of beneficial gut microbiota, and promoting the intestinal integrity [89]. Addition of apple pulp up to 10% with multi-enzyme additive at 0.05% resulted in improved laying performance, blood parameters, and egg traits of hen without affecting other traits [90]. Supplementation of enzymes in diets with low energy resulted in up-regulated expression of nutrient transporters that enhanced the micronutrient absorption and growth performance of broiler chickens [87]. Because of their versatile and positive effects on poultry production, enzymes and enzyme-based products are the best alternatives to antibiotics, which also guarantee food safety.
Although enzymes can enhance the performance of chickens, they are temperature sensitive, and heating reduces their effectiveness during feed processing. The high cost also limits their practical applications on a large scale in comparison to traditional additives.
3.5. Prebiotics
There have been huge concerns related to the presence of antimicrobial residues in the environment, also in meat, primarily due to usage in animal husbandry, therefore the world is trying to explore alternatives to veterinary antibiotics. One such alternative is probiotics, intended to reduce or replace the antimicrobial usage [91]. Prebiotics are oligosaccharides carbohydrates, made up of 2-10 monosaccharides [92] that are utilized by the microorganisms in the intestine but are not digestible by the host animal, including mannan oligosaccharides, oligofructose, fructooligosaccharide, galactan, galactooligosaccharides, inulin, and fiber components [54]. It is considered that prebiotics and probiotics result in a change of the composition of the normal microflora of the intestine towards beneficial microflora from a potentially harmful composition and thus benefit the host [54, 93]. Saccharomyces cerevisiae supplementation in the feed improved the efficiency, feed digestibility, increased the animal performance, reduced the pathogenic bacteria in numbers, and improved animal health [94]. Mannan oligosaccharides supplementation in the poultry feed enhanced the growth performance, oxidative status of the bird, immunoglobulin content, improved serum biochemical profile and decreased the cecal salmonella colonies [79, 95].
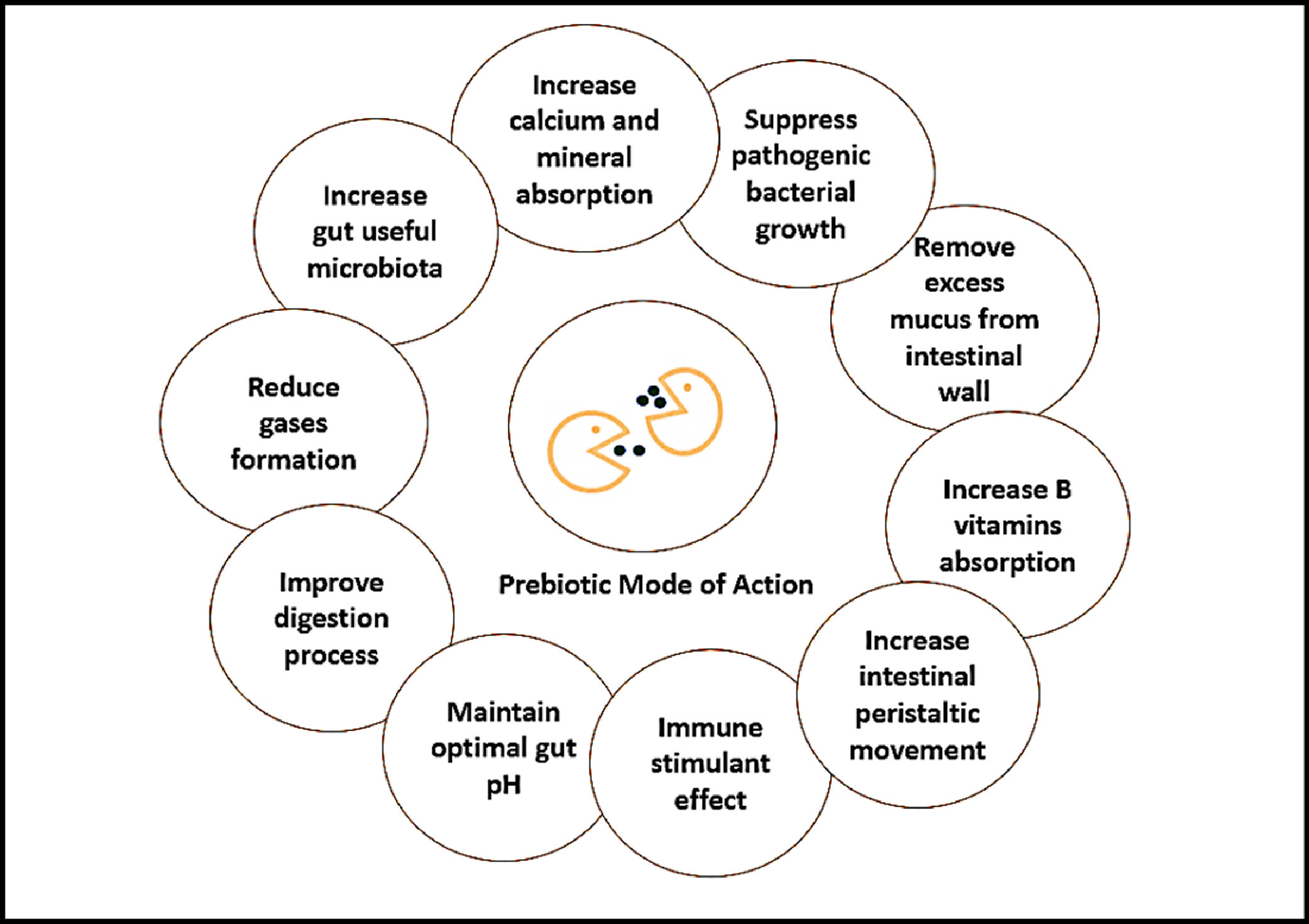
Figure 2. Prebiotics mode of action. Probiotics mode of action. Reproduced from Abd El-Hack et al. [48] under the Creative Commons BY-NC-ND 4.0 license.
3.6. Probiotics
Direct fed microbials or probiotics can be described as living, beneficial microbial feed supplements, such as bacteria (Bifidobacteria, Bacilli, Lactobacilli), fungi (Aspergillus awamori and Aspergillus oryzea) and yeast (Saccharomyces) that results in the microbial balance in the intestine and increase the intestinal health, immune response and thus improve poultry performance [54]. Probiotics impart many beneficial effects on performance, intestinal microbiota modulation, pathogens inhibition, improvement of intestinal integrity, immunomodulation, and improvement of microbiological and sensory characteristics of poultry meat [48]. Probiotics are an ideal alternative to antibiotics, but they struggle with strain-specific efficacy and need accurate selection to match the target pathogens. Their persistence also reduces during competition with the native microbiota in the GIT [96]. Due to no standardization in the dosage, prebiotics can also promote harmful bacteria and precise attention is required during their formulation [96] But probiotics further need to be addressed.
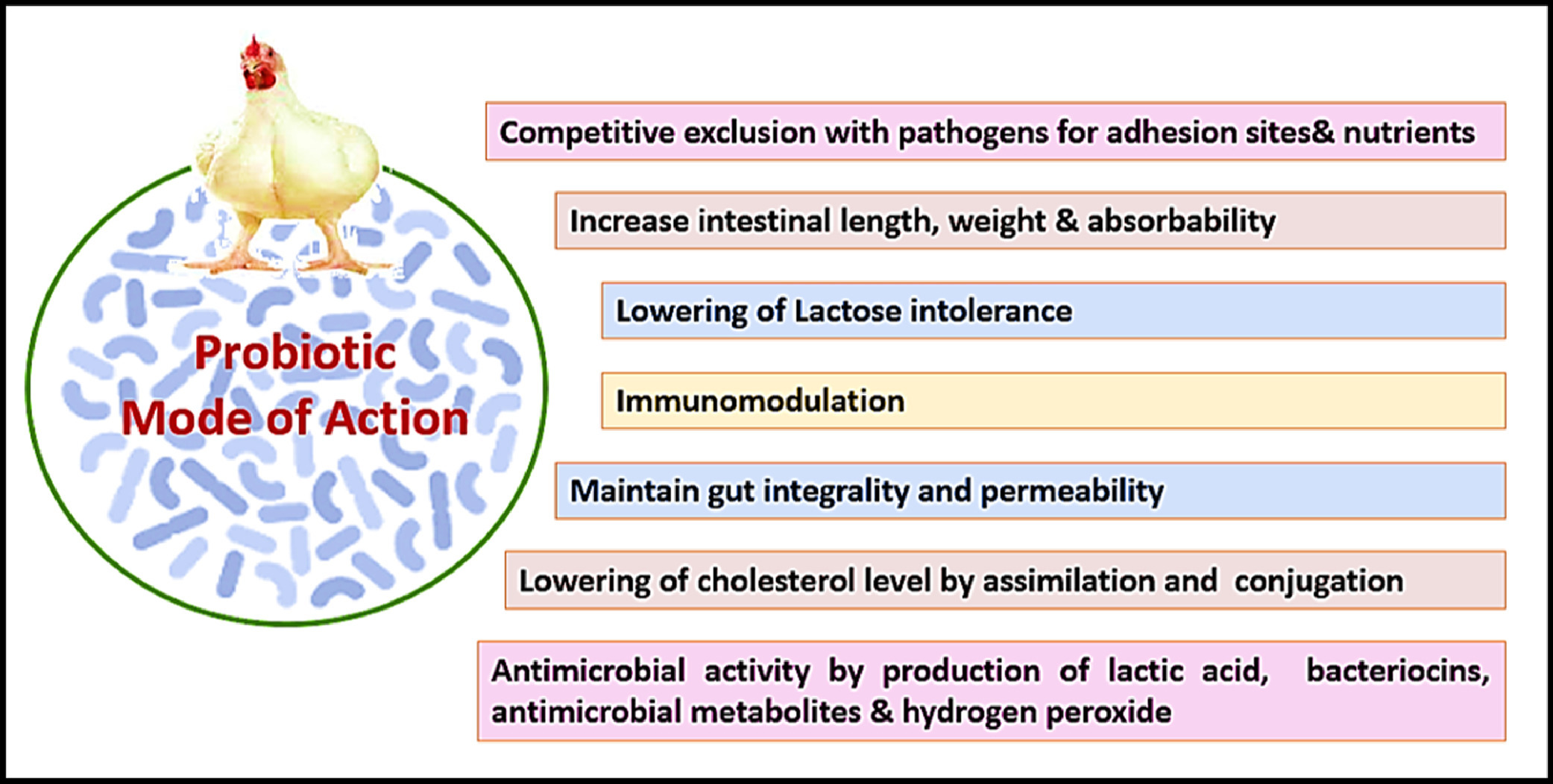
Figure 3. Probiotics mode of action. Reproduced from Abd El-Hack et al. [48] under the Creative Commons BY-NC-ND 4.0 license.
3.6.1. Probiotics Bacteria
Probiotics bacteria can produce and release antimicrobial molecules like organic acid compounds, diacetyl, hydrogen peroxide, and peptides, vitamins, amino acids, teichoic acids, peptidoglycans that have the potential to work selectively against various microbial strains that are found in the gut. Bacterial AMPs are also called bacteriocins, a diverse class of ribosomal synthesized peptides that have a role in the direct elimination of bacteria or cessation of their growth in the lumen [97]. According to a study by Abd el-Moneim et al. [98] with in ovo inoculation of inoculation of Bifidobacterium Bifidum, Bifidobacterium animalis, Bifidobacterium longum and Bifidobacterium infantis, resulted in improved ileal bacterial composition with increased colonization of the intestines with bacterial species such as Lactobacillus and Bifidobacterium and decreased the overall Coliform bacteria. The inclusion of Bacillus Toyonesis and Bacillus bafidium in the diet of quail, hindered the growth of fungi, E. coli and coliforms in the cecum [99]. Different Bacillus species are used by the poultry industry that are performing beneficial effects as shown in (Table 3) [100].
Table 3. Usage of probiotic bacteria in the poultry industry.
| Probiotics | Biological Performance | References |
|---|---|---|
| Lactiplantibacillus plantarum LTC-113 | Strengthened resistance against Salmonella typhimurium and maintained the integrity of the intestinal epithelial barrier | [101, 102] |
| Lactobacillus johnsonii | Reduced the symptoms of Clostridium perfringens and Salmonella sofia infections | [103, 104] |
| Bacillus subtilis C-3102 | Reduced the level of Salmonella enterica serovars (enteritidis LM-7) | [105, 106] |
| Pediococcus acidilactici | Number of Salmonella enterica serovars (Gallinarum) got reduced | [107, 108] |
| Lactobacillus acidophilus | Increased the immune response, reduced mortality and increased the body weight of hens challenged with Escherichia coli O157 | [109, 110] |
| Bacillus subtilis | Increased the level of advantageous bacteria (Lactobacillus, Bifidobacterium, and Enterococcus), increased the level of Blautia, Faecalibacterium, and Romboutsia, resulted in the increment of surface area availability for absorption in the duodenum and ileum with an increased ratio of villus height to crypt depth | [111, 112] |
| Bacillus subtilis PB-6 | Enhanced plasma calcium and phosphorous concentrations, improved bone mass and meat quality with productivity and welfare | [113, 114] |
| Bacillus subtilis DSM29784 | Boost the quantity of Butyricicoccus and Faecalibacterium in the gut, enhances the tight junction complex, weight and overall health in broilers | [115, 116] |
3.6.2. Fungi as Probiotics
Aspergillus spp. is the most predominant in the intestinal tract and cecum of poultry birds, indicating viability and importance as a probiotic [117]. Different species of fungi like Aspergillus awamori, A. niger, and A. oryza have the potential to maintain the intestinal microflora balance and enhance the immune response [100]. A study suggests that the addition of 0.2% Aspergillus meal in the diet of chicken may have effects in reducing the Salmonella spp, levels and increase the food safety of meat [118]. Addition of Aspergillus awamori in the diet of growing rabbits at 100-150 mg/kg diet results in enhanced growth, intestinal health, nutrient digestibility, immune status and antioxidative responses [119]. Supplementation of Aspergillus niger in the diet of egg-laying hen (Hy-Line W-36) at a rate of 220 g/kg feed resulted in a significant increase in egg production, Haugh unit and egg quality. However, this lowers the microbial load of pathogenic microbiota i.e., Clostridium perfringens, Salmonella spp., and Escherichia coli, in the caeca [117]. According to a study by Zahirian et al., [120] that addition of Aspergillus oryza in the diet of broilers resulted in an increment of weight gain, reduction in the proportion of abdominal fat, aspartate aminotransferase and alanine aminotransferase levels in the serum, and antibody titters against Avian influenza and Newcastle disease. Due to these benefits fungi as a probiotic may be a better alternative to antibiotics in poultry.
3.6.3. Yeast Probiotics
Yeasts are eukaryotic unicellular fungi that are ubiquitous in nature and found in the environment, including soil and the skin of animals and humans [121]. Their cell wall components like mannans (40%), chitin (2%), and glucans (60%), as well as the result of yeast hydrolysis like nucleotides, vitamins, and other compounds, have been shown to improve poultry production performance, gastrointestinal health, and immune system [122]. It was observed in a study that P. guilliermondii exhibited the greatest resistance to gastric juice, moderate enzymatic activity, antimicrobial activity, and increased adhesion to cells [123]. Similarly, the supplementation of yeast probiotics increases the chicken’s resistance to enteric pathogens like Salmonella, Campylobacter jejuni, C. perfringens, or E. coli. Administration of 3g/ ton of feed of S. cereveciae in broilers exhibited lower blood cholesterol concentrations in a study by Pang et al., [124]. Therefore, yeast probiotics emerge as an excellent alternative to antibiotics.
The manufacturing of yeast probiotics is quite complicated and needs advanced bioprocessing techniques like high-cell-density cultivation, which limits their wide applicability. The shelf life is also compromised in case of the storage of oxygen-sensitive strains [125].
4. Nanoparticles (NPs) and Nanotechnology Applications
Nanotechnology is a promising and innovative technology with wide biomedical utilization and potential implementations in the poultry industry [126]. Nanoparticles (NPs) generally in between 1 and 100 nm also called nanobiotics have antimicrobial properties and are among the latest alternatives to deal with multi-drug-resistant pathogens [127]. NPs are classified into four categories like inorganic, organic, carbon base and hybrid. Inorganic groups include metal, metal oxide nanoparticles, and quantum dots. However, organic nanomaterials consist of biocompatible organic components such as lipid-based nanoparticles, polymeric nanoparticles, liposomes and because of these they are widely used for drug delivery, as antimicrobials and in the regeneration of tissues. Furthermore, carbon-based nanomaterials include nanofibers, nanodots, carbon onions, carbon rings, etc. Inorganic nanoparticles consist of inorganic oxides of Si, Ag, Au, Zn, Mg, Mn, Cu, Se, Al, and Ti and show differences in shape, size, solubility, and stability. In addition to this, their potency is also affected by the pH, temperature, concentration of reducing agent, and reduction time [128]. In comparison to traditional nanoparticles, iron oxide nanoparticles (IO-NPs) and zinc oxide nanoparticles (ZnO-NPs) exhibit stronger bactericidal effects due to the reduced ion particle sizes and high surface energies [129]. An in-vitro study with ZnO-NPs usage revealed its potential as an antibacterial agent in poultry production in reducing the load of foodborne pathogens in the gut of poultry and found S. aureus as the most susceptible bacteria [127]. ZnO-NPs as a feed supplement are helpful in reducing footpad dermatitis that is induced by multidrug-resistant Staphylococcus aureus (MRSA) in broiler birds [126]. Usage of Fe3O4-NPs pretreatment restricted the invasion of S. Enteritidis in the liver of chicken and significantly increased the reactive oxygen species (ROS) in chickens [130]. In another study, Ali and Bakheet [131] concluded that in ovo inoculation of chitosan-NPs reduced the E. coli count without harming the hatchability and showed the highest inhibitory effect on the biofilms forming E.coli strains. Antibacterial mechanisms of some NPs are still uncertain, and more studies are needed to determine their future applications. Therefore, potential NPs usage as antimicrobial agents in the poultry sector needs further investigation.
There are safety concerns in the application of nanotechnology as inorganic NPS (Si, Ag, Au, Zn, Mg, etc.) can accumulate in the poultry tissues. The cost of production and possible degradation in the GIT remain economically unviable for large-scale use. More research is needed to standardize their dosage and temperature stability during the pelleting of feed [132].
4.1. NPs-Mechanism of Action
Nanoparticles exhibit their antimicrobial properties by utilizing different modes of action depending on the composition and bacterial interaction. NPs can perform their action by disrupting the bacterial cell membrane integrity. Inorganic nanoparticles (AgNPs, CuNPs, IO-NPs, ZnO-NPs) can generate reactive oxygen species (ROS) that result in oxidative damage to bacterial membranes and weaken their structure [133]. Metal ions interact with the membrane protein, resulting in an increase in the permeability of the membrane, causing the cellular contents to leak out and ultimately the lysis of the cell [134]. Quantum dots also create ROS under UV/Visible light and causing oxidation of DNA and protein damage [135]. Metal and metal oxide nanoparticles also present a unique mechanism by inhibiting the bacterial communication (quorum sensing) involved in behavior like biofilm formation and virulence, by the degradation of acyl-homoserine lactones (AHLs), a signaling molecule [136, 137]. Lipid-polymeric NPs have the ability to encapsulate the bioactive compounds, antimicrobial drug and then release them on the target site to reduce the load of bacteria with enhanced efficacy [138, 139]. Moreover, carbon-based nanoparticles penetrate the bacterial cell wall mechanically and causing physical damage and the loss of integrity and also by creating oxidation of vital cellular structures [140]. Furthermore, carbon-based nanoparticles also prevent the formation of biofilm by adsorption of extracellular polymeric substances(EPS) via electrostatic and hydrophobic interactions, thus weakening bacterial adhesion [141]. In this way, nanoparticles exhibit their potential to work effectively as antimicrobials.
5. One Heath Approach to Antimicrobial Resistance
One health is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems. Bacteriophages have spe-cies-specific activities against pathogens and reduce zoonotic transmission of multi-drug-resistant pathogens like Salmonella and methicillin-resistant Staphylococcus aure-us (MRSA)[142, 143]. Their natural presence in environment supports ecological bal-ance and aligns with One Health approach [143]. Similarly, AMPs offer biodegradable solutions to overcome resistance issues through nonspe-cific membrane disruption. They reduce antibiotic residues in meat products, thereby decreasing human exposure to resistant bacteria through the food chain [144]. AMPs also degrade rapidly in the ecosystem and reduce contamination issues linked to tradi-tional antibiotics [145]. Moreover, feed additives such as botanical nutraceuticals con-tribute to the one health approach by enhancing poultry health and reducing the need for antibiotics. Botanical products like fenugreek seeds and ginger roots improve diges-tive enzyme activity and restore microbiota balance, which minimizes the risk of an-timicrobial resistance. These additives also support environmental sustainability with no chemical residues [49]. Enzymes such as phytase and xylanase improve nutrient digestibility in poultry feed, reducing phosphorus excretion and environmental pollu-tion [146]. Additionally, enzyme-based nanomotors are promising bactericidal agents against pathogens like Escherichia coli and offer targeted antimicrobial effects without contribution of microbial resistance [147]. Probiotics and prebiotics enhance poultry gut microbiota and reduce antibiotic use in poultry. This directly impacts human health by lowering the antibiotic-resistant enteric pathogens in food animals, which are estimated to cause 10 million annual human deaths by 2050 if unaddressed [148, 149]. Fungal probiotics, such as those derived from Arthrospira platensis, modulate gut microbiota and provide immunomodulatory benefits. They fit within the One Health framework and improve poultry health through sustainable means. They also enhance feed conversion efficiency, reduce resource consumption and mitigate environmental stressors associated with intensive poultry farming [150]. Yeast probiotics improve gut health by inhibiting bacterial colonization and enhancing immune responses. Their ability to modulate intestinal microbiota aligns with One Health principles by reducing zoonotic transmission risks of resistant pathogens like Salmonella [151]. Besides that, nanotechnology offers innovative solutions for AMR mitigation through encapsulated probiotics, antioxidants and enzyme-based nanomotors. Nanoencapsulation improves probiotic stability in harsh gut environments, enhancing their efficacy against patho-gens like Campylobacter jejuni. This approach interrupts pathogen transmission cycles through the food chain, protecting human health while promoting sustainable agri-cultural practices [152].
6. Future Research Direction
Exploration of sustainable alternatives to antibiotics in poultry farming is valuable to enhance animal health and reduce antimicrobial resistance. Bacteriophages have gained popularity as antibacterial agents against Salmonella and E. coli in poultry pathogens, and future studies should focus on genetic modification to enhance stability and effectiveness. Moreover, the creation of a broad-spectrum phage cocktail should be considered. There is a need to establish a regulatory framework to boost its commercialization [63]. Similarly, AMPs have been investigated as a natural alternative with broad-spectrum activity. Future research should consider the synthetic AMPs with greater stability by utilizing better encapsulation techniques with stabilizers to protect them from degradation. In the feed additives, research should aim at elucidating their exact mechanisms of action and the development of standardized formulations with more exploration in synergistic interactions with other feed additives [153]. However, there is a need for studies to explore new enzymes, with increased efficiency through the usage of genetic engineering and to investigate how they interact in the gut with microbiota in a more precise manner. Strategies to reduce the cost of production of enzymes and enzyme-based products should be considered. Probiotics and prebiotics have shown their positive role in modulating the gut microbiota, and in future, they would be more beneficial in poultry farming by having a major focus on identifying strains with specific health benefits, and the development of optimized formulations with increased efficacy. Large scale field trials are also needed to confirm their effectiveness in commercial settings [2]. Nanoparticles with antimicrobial and delivery potential should be studied more to assess their safety and appropriate levels to prevent toxicity.
7. Conclusion
In the poultry industry, bacterial infections are one of the major causes of morbidity and mortality throughout the world. Traditionally, antibiotics were used to treat bacterial infection and as a growth promoter for optimum performance, but this results in the antibiotic resistance in bacteria and many strains of bacteria became resistant to antibiotics even at very high doses. Therefore, to deal with this global antimicrobial resistance (AMR) issue and to maintain the health of poultry birds, many sustainable strategies are under consideration with limited or no usage of antibiotics which foster the health and immune status of birds. Feed additives, prebiotics and probiotics are gaining popularity in the poultry industry due to greater performance and easy management. However, bacteriophage, bacteriophage, phytobiotic/medicinal plants extracts, and nanoparticle-based alternatives are considered as emerging alternatives as they showed great inhibitory effects against microorganisms and are helpful against multi-drug-resistant pathogens. There is also huge concern related to finding potential alternatives to antibiotics as there are many alternatives. Despite the benefits, all the alternative approaches also have some limitations in their potential as antimicrobial, and safety. However, the poultry industry is still lagging and has a scarcity of innovative investigations and therefore, further research should be done to slow down the development of multidrug- resistant strains of bacteria. According to research, natural alternatives to antibiotics as growth promoters are recommended in the poultry industry as they are safe and healthy and impart positive effects on the immune system. Essential oils are volatile, so they should be used by having proper encapsulation, which enhances bioactivity and stability. These natural alternatives also result in productivity enhancement, enhanced digestion, improved nutrient availability, absorptivity and reduced resistance to antibiotics with the production of safe meat and eggs, but still there is a need to identify and characterize new natural alternatives to strengthen this industry.
References
- Worldometer. Current world population. Available at: [online link] (accessed on January 10, 2025).
- Kleyn, F.; Ciacciariello, M. Future demands of the poultry industry: will we meet our commitments sustainably in developed and developing economies? World's Poult. Sci. J. 2021, 77 (2), 267–278. [Google Scholar] [CrossRef]
- Searchinger, T.; Hanson, C.; Ranganathan, J.; Lipinski, B.; Waite, R.; et al. Creating a sustainable food future: a menu of solutions to sustainably feed more than 9 billion people by 2050. World Resources Report 2013–14: Interim Findings; World Resources Institute: Washington, DC, 2014; 154 p. ISBN 978-1-56973-817-7. [DOI]
- WHO. 5 tips for a healthy diet this new year. Available at: [online link] (accessed on January 10, 2025).
- Kumar, D.; Pornsukarom, S.; Thakur, S. Antibiotic usage in poultry production and antimicrobial-resistant Salmonella in poultry. In Food safety in poultry meat production; 2019; pp 47–66. [Google Scholar]
- Selaledi, A. L.; Mlambo, V.; Masika, P. J.; Dlamini, N. R.; Dube, B. The current status of the alternative use to antibiotics in poultry production: an African perspective. Antibiotics 2020, 9 (9), 594. [Google Scholar] [CrossRef]
- Islam, K. S.; Shiraj-Um-Mahmuda, S.; Hazzaz-Bin-Kabir, M. Antibiotic usage patterns in selected broiler farms of Bangladesh and their public health implications. J. Public Health Dev. Ctries. 2016, 2 (3), 276–284. [Google Scholar]
- Haque, M. H.; Sultana, S.; Rahman, M. H.; Islam, M. R. Sustainable antibiotic-free broiler meat production: current trends, challenges, and possibilities in a developing country perspective. Biology 2020, 9 (11), 411. [Google Scholar] [CrossRef]
- Hedman, H. D.; Vasco, K. A.; Zhang, L. A review of antimicrobial resistance in poultry farming within low-resource settings. Animals 2020, 10 (8), 1264. [Google Scholar] [CrossRef]
- Göransson, L.; Yngvesson, J.; Gunnarsson, S. Bird health, housing and management routines on Swedish organic broiler chicken farms. Animals 2020, 10 (11), 2098. [Google Scholar] [CrossRef]
- Medina-Paredes, A. C.; Paredes-Peralta, M. M.; Varga, E. Assessing the sectoral and cross-sectoral impacts of new European Union broiler chicken welfare measures in Hungary as proposed by the European Food Safety Authority. Stud. Agric. Econ. 2024, 126 (1). [Google Scholar] [CrossRef]
- Nicolae, C. G.; Popescu, A.; Voicu, A.; Constantinescu, R. EU regulations for organic aquaculture—a key for producing organic food. Presented at the 32nd IBIMA Conference, Seville, Spain, 2018. [Google Scholar]
- Brunberg, E.; Grøva, L.; Serikstad, G. L. Genetics and welfare in organic poultry production: a discussion on the suitability of available breeds and hybrids. Bioforsk Rapp. 2014, 9 (143). [Google Scholar]
- Castellini, C.; Bastianoni, S.; Dal Bosco, A.; Brunetti, M.; Mugnai, C. Adaptation to organic rearing system of eight different chicken genotypes: behaviour, welfare and performance. Ital. J. Anim. Sci. 2016, 15 (1), 37–46. [Google Scholar] [CrossRef]
- Göransson, L.; Lundmark Hedman, F. The perks of being an organic chicken–animal welfare science on the key features of organic poultry production. Front. Anim. Sci. 2024, 5, 1400384. [Google Scholar] [CrossRef]
- Williams, C. In ovo vaccination and chick quality. Int. Hatch. Prac. 2011, 19, 7–13. [Google Scholar]
- Bal, A. In ovo vaccination against coccidiosis. In Proceedings of the 5th Mediterranean Poultry Summit of the WPSA, 2009. [online link]
- Peebles, E. In ovo applications in poultry: a review. Poult. Sci. 2018, 97 (7), 2322–2338. [Google Scholar] [CrossRef]
- Kucharska-Gaca, J.; Kowalska, E.; Dębowska, M. In ovo feeding—technology of the future—a review. Ann. Anim. Sci. 2017, 17 (4), 979–993. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, R. Methods for early nutrition and their potential. World's Poult. Sci. J. 2004, 60 (1), 101–111. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Food safety and quality: biosecurity. [online link] (accessed on January 10, 2024).
- Militzer, N.; McLaws, M.; Rozstalnyy, A.; Li, Y.; Dhingra, M.; Auplish, A.; Mintiens, K.; Sabirovic, M.; von Dobschuetz, S.; Heilmann, M. Characterising biosecurity initiatives globally to support the development of a progressive management pathway for terrestrial animals: a scoping review. Animals 2023, 13 (16), 2672. [Google Scholar] [CrossRef]
- Souillard, R.; Allain, V.; Dufay-Lefort, A. C.; Rousset, N.; Amalraj, A.; Spaans, A.; Zbikowski, A.; Piccirillo, A.; Sevilla-Navarro, S.; Kovács, L.; Le Bouquin, S. Biosecurity implementation on large-scale poultry farms in Europe: a qualitative interview study with farmers. Prev. Vet. Med. 2024, 224, 106119. [Google Scholar] [CrossRef]
- Bao, T. D.; Van Cuong, N.; Phu, D. H.; Dung, N. T. T.; Kiet, B. T.; Rushton, J.; Carrique-Mas, J. Economic assessment of an intervention strategy to reduce antimicrobial usage in small-scale chicken farms in Vietnam. One Health 2024, 18, 100699. [Google Scholar] [CrossRef]
- Ravikumar, R.; Chan, J.; Prabakaran, M. Vaccines against major poultry viral diseases: strategies to improve the breadth and protective efficacy. Viruses 2022, 14 (6), 1195. [Google Scholar] [CrossRef]
- Islam, M. S.; Rahman, M. T. A comprehensive review on bacterial vaccines combating antimicrobial resistance in poultry. Vaccines 2023, 11 (3), 616. [Google Scholar] [CrossRef]
- Khair, S. M.; Saifuddin, M.; Jahan, S.; Ahmed, R. U.; Rahman, M. T. Preparation, experimental and molecular evaluation of fowl cholera chicken embryo derived inactivated bacterin. J. Adv. Vet. Res. 2024, 14 (1), 218–222. [Google Scholar]
- Gasimov, R.; Sadigova, G.; Mammadov, I. Effectiveness of the study of plant-containing immunostimulants in poultry industry. Sch. Bull. 2023, 9 (6), 76–79. [Google Scholar]
- Esan, O.; Abubakar, M. B.; Abdulrahman, M. A.; Yusuf, I. O.; Muhammad, M. B.; Muhammad, A. A.; Bello, M. B. Immunostimulating potentials of methanolic extract of Plectranthus parviflorus in chickens vaccinated against Newcastle disease and infectious bursal disease. Sokoto J. Vet. Sci. 2023, 21 (4), 208–215. [Google Scholar]
- Hegazy, A. M.; Salem, H. M.; Mostafa, A. A.; Sayed-Ahmed, M. Z.; Elbaz, A. M.; El-Sharkawy, H. Evaluation of the immuno-stimulatory effect of aqueous neem (Azadirachta indica) leaf extract against highly pathogenic avian influenza (H5N8) in experimental chickens. Poult. Sci. 2023, 102 (11), 103043. [Google Scholar] [CrossRef]
- Darwish, A. M. F.; El-Saadony, M. T.; Saad, A. M.; El-Wafai, N. A.; El-Far, A. H. Comparative study between mushroom-extracted and commercial lectins: impact of immune response to H5N1, NDV, and IBD vaccines in broiler chicken. Benha J. Appl. Sci. 2024, 9 (3), 113–120. [Google Scholar] [CrossRef]
- Jiang, A.; Yang, X.; Yang, X.; Zhang, Y.; Yang, H. Prospects and challenges of bacteriophage substitution for antibiotics in livestock and poultry production. Biology 2024, 13 (1), 28. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Guideline on Quality, Safety and Efficacy of Veterinary Medicinal Products Specifically Designed for Phage Therapy; European Medicines Agency: London, 2024. [Online] (accessed June 2024).
- Zeng, Y.; Wang, Z.; Zou, T.; Chen, J.; Li, G.; Zheng, L.; Li, S.; You, J. Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front. Vet. Sci. 2021, 8, 623899. [Google Scholar] [CrossRef]
- Kim, J. S.; Hosseindoust, A.; Lee, S. H.; Choi, Y. H.; Kim, M. J.; Lee, J. H.; Kwon, I. K.; Chae, B. Bacteriophage cocktail and multi-strain probiotics in the feed for weanling pigs: effects on intestine morphology and targeted intestinal coliforms and Clostridium. Animal 2017, 11 (1), 45–53. [Google Scholar] [CrossRef]
- Abd-El Wahab, A.; Elbestawy, A. R.; Ghanem, H. M.; Abd El-Hack, M. E.; Khafaga, A. F.; Noreldin, A. E. An overview of the use of bacteriophages in the poultry industry: successes, challenges, and possibilities for overcoming breakdowns. Front. Microbiol. 2023, 14, 1136638. [Google Scholar] [CrossRef]
- Naiel, M. A.; Ismael, N. E.; Abd El-Hack, M. E.; Amer, M. S.; Khafaga, A. F. Applications of antimicrobial peptides (AMPs) as an alternative to antibiotic use in aquaculture - a mini-review. Ann. Anim. Sci. 2023, 23 (3), 691–701. [Google Scholar] [CrossRef]
- Büyükkiraz, M. E.; Kesmen, Z. Antimicrobial peptides (AMPs): a promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132 (3), 1573–1596. [Google Scholar] [CrossRef]
- Xu, Y.; Lai, R.; Zhang, Y.; Liu, H.; Lin, Y. Evaluation of the efficacy of the antimicrobial peptide HJH-3 in chickens infected with Salmonella Pullorum. Front. Microbiol. 2023, 14, 1102789. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial peptides: an alternative to antibiotic for mitigating the risks of antibiotic resistance in aquaculture. Environ. Res. 2024, 251, 118619. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, W.; Zhu, M.; Wang, J.; Lu, H.; Liu, L. Effects of antimicrobial peptide microcin C7 on growth performance, immune and intestinal barrier functions, and cecal microbiota of broilers. Front. Vet. Sci. 2022, 8, 813629. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z.; Xu, Y.; Wang, X. Effect of antimicrobial peptide microcin J25 on growth performance, immune regulation, and intestinal microbiota in broiler chickens challenged with Escherichia coli and Salmonella. Animals 2020, 10 (2), 345. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zhao, J.; Wu, J.; Tang, Z. The effect of the antimicrobial peptide plectasin on the growth performance, intestinal health, and immune function of yellow-feathered chickens. Front. Vet. Sci. 2021, 8, 688611. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers. J. Anim. Sci. 2015, 93 (10), 4750–4760. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Lin, Q.; Zhao, J.; Feng, Y. Effects of dietary supplement of ε-polylysine hydrochloride on laying performance, egg quality, serum parameters, organ index, intestinal morphology, gut microbiota and volatile fatty acids in laying hens. J. Sci. Food Agric. 2024, 104 (5), 3069–3079. [Google Scholar] [CrossRef]
- Jodoin, J.; Hincke, M. T. Histone H5 is a potent antimicrobial agent and a template for novel antimicrobial peptides. Sci. Rep. 2018, 8 (1), 2411. [Google Scholar] [CrossRef]
- Lima, L. F.; Rolim, G. S.; Costa, M. P.; Freire, C. P. Application of antimicrobial peptides in the poultry industry. Vet. Microbiol. 2024, 285, 110267. [Google Scholar] [CrossRef]
- Abd El-Hack, M. E.; Alagawany, M.; Arif, M.; Soomro, R. N.; Greish, H. E. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022, 101 (4), 101696. [Google Scholar] [CrossRef]
- El-Sabrout, K.; Khalifah, A.; Mishra, B. Application of botanical products as nutraceutical feed additives for improving poultry health and production. Vet. World 2023, 16 (2), 369–379. [Google Scholar] [CrossRef]
- Elbaz, A. M.; Salama, A.; Khidr, R.; Ibrahim, D. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet. Res. 2022, 18 (1), 430. [Google Scholar] [CrossRef]
- Akbari, A.; Jelodar, G.; Nazifi, S.; Sajedianfard, J. An overview of the characteristics and function of vitamin C in various tissues: relying on its antioxidant function. Zahedan J. Res. Med. Sci. 2016, 18 (11), e4037. [Google Scholar] [CrossRef]
- Bechara, N.; Flood, V. M.; Gunton, J. E. A systematic review on the role of vitamin C in tissue healing. Antioxidants 2022, 11 (8), 1605. [Google Scholar] [CrossRef]
- Panda, A. K. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of White Leghorn layers under tropical summer conditions. Br. Poult. Sci. 2008, 49 (5), 592–599. [Google Scholar] [CrossRef]
- El-Baz, A.; Khidr, R. Role of feed additives in poultry feeding under marginal environmental conditions. In Feed Additives: Recent Trends in Animal Nutrition; IntechOpen: London, 2024. [Google Scholar] [DOI]
- Elbaz, A. M.; El-Baz, A.; El-Sayed, R.; Hassan, M. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021, 53 (1), 115. [Google Scholar] [CrossRef]
- Mahmoud, H.; Afiffy, O.; Mahrous, M. Effect of using formic acid on growth performance and some blood parameters of broiler chicken. Assiut Vet. Med. J. 2020, 66 (164), 140–154. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Yang, H.; Zhang, Q. Effects of replacing in-feed antibiotics with synergistic organic acids on growth performance, health, carcass, and immune and oxidative statuses of broiler chickens under Clostridium perfringens type A challenge. Avian Dis. 2020, 64 (3), 393–400. [Google Scholar] [CrossRef]
- Dilawar, M. A.; Khan, A.; Ahmad, N.; Ali, M. Egg quality parameters, production performance and immunity of laying hens supplemented with plant extracts. Animals 2021, 11 (4), 975. [Google Scholar] [CrossRef]
- Marume, U.; Mlambo, V.; Hugo, A.; Marume, A. Citrullus lanatus essential oils inclusion in diets elicit nutraceutical effects on egg production, egg quality, and physiological characteristics in layer hens. Poult. Sci. 2020, 99 (6), 3038–3046. [Google Scholar] [CrossRef]
- Aminullah, N.; Hussain, J.; Ijaz, M.; Mehmood, K. Phytogenic feed additives as alternatives to antibiotics in poultry production: a review. Vet. World 2025, 18 (1),141–154. [Google Scholar] [CrossRef]
- Dennehy, J. J.; Abedon, S. T. Phage infection and lysis. In Bacteriophages: Biology, Technology, Therapy; Harper, D. R., Abedon, S. T., Burrowes, B. H., McConville, M. L., Eds.; Springer: Cham, Switzerland, 2021; pp 341-383. [Google Scholar] [DOI]
- Jia, H.-J.; Yang, Y.-Z.; Liu, Y.; Wang, Y. Engineering bacteriophages for enhanced host range and efficacy: insights from bacteriophage-bacteria interactions. Front. Microbiol. 2023, 14, 1172635. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10 (5), 872. [Google Scholar] [CrossRef]
- Joerger, R. D. Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003, 82 (4), 640-647. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22 (21), 11401. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Pustijanac, E.; Škrlec, I. Antimicrobial peptides-mechanisms of action, antimicrobial effects and clinical applications. Antibiotics 2022, 11 (10), 1417. [Google Scholar] [CrossRef]
- Nazeer, N.; Khan, S.; Yousaf, A.; Rasool, M. H. Antimicrobial peptides as an alternative to relieve antimicrobial growth promoters in poultry. Br. Poult. Sci. 2021, 62 (5), 672-685. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, M.; Huang, X.; Wu, Y. Development strategies and application of antimicrobial peptides as future alternatives to in-feed antibiotics. Sci. Total Environ. 2024, 926, 172150. [Google Scholar] [CrossRef]
- Choi, S.; Ingale, S. L.; Kim, J. S.; Park, Y. K.; Kwon, I. K.; Chae, B. J. An antimicrobial peptide-A3: effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Br. Poult. Sci. 2013, 54 (6), 738-746. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Rose, S. P.; Ivanova, S. Feed Additives in Poultry Nutrition; CABI: Wallingford, UK, 2019; pp 1-200. [Google Scholar]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J. F. Phytogenic feed additives in poultry: achievements, prospective and challenges. Animals 2021, 11 (12), 3471. [Google Scholar] [CrossRef]
- Hussain, R.; Akhtar, M.; Khan, A. Mitigation of enteric pathogens and modulation of gut microbiota in livestock by natural feed additives. In Complementary and Alternative Medicine: Feed Additives; Gupta, R. C., Srivastava, A., Lall, R., Eds.; Academic Press: London, 2022; pp 125-138. [Google Scholar]
- Omer, H. A.; Fayed, A.; Abou-Elkhair, R. Nutritional impact of inclusion of garlic (Allium sativum) and/or onion (Allium cepa L.) powder in laying hens' diets on their performance, egg quality, and some blood constituents. Bull. Natl. Res. Cent. 2019, 43 (1), 23. [Google Scholar] [CrossRef]
- Selle, P. H.; Ravindran, V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007, 135 (1-2), 1-41. [Google Scholar] [CrossRef]
- Khattak, F.; Pasha, T. N.; Hayat, Z. Enzymes in poultry nutrition. J. Anim. Plant Sci. 2006, 16 (1-2), 1-7. [Google Scholar]
- Bansal, S.; Goel, G.; Ojha, S. Applications of industrially important enzymes. In Industrial Enzymes; Shukla, P., Ed.; Academic Press: London, 2022; pp 49-62. [Google Scholar]
- da Silva, I. C.; de Oliveira, M. C.; Albino, L. F. T.; de Souza, J. B.; Rostagno, H. S. Dry residue of cassava associated with carbohydrases in diets for broiler chickens. J. Appl. Poult. Res. 2019, 28 (4), 1189-1201. [Google Scholar] [CrossRef]
- Binns, N. Probiotics, Prebiotics and the Gut Microbiota; ILSI Europe: Brussels, Belgium, 2013; pp 1-32. [Google Scholar]
- Zhou, M.; Duan, Z.; Dong, Y.; Zeng, Z. Effects of mannanoligosaccharide supplementation on the growth performance, immunity, and oxidative status of Partridge Shank chickens. Animals 2019, 9 (10), 817. [Google Scholar] [CrossRef]
- Kadam, J. H.; Mehta, A. N.; Nair, S. S. Advances on probiotics utilization in poultry health and nutrition. In Advances in Probiotics for Health and Nutrition; Rai, A. K., Bai, J. A., Eds.; IntechOpen: London, 2023; pp 1-18. [Google Scholar] [CrossRef]
- Khaneghah, A. M.; Abedi, A.; Eş, et. al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205-218. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, C.; Zhang, H.; Fan, Y.; Zhang, Y.; Zhan, X. Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals 2019, 9 (12), 1110. [Google Scholar] [CrossRef]
- Das, B.; Dash, S. K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Dey, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10 (6), 862-876. [Google Scholar] [CrossRef]
- Abreu, R.; Barbosa, A. V.; Carrilho, E.; Perugini, M. R. E. Antimicrobial drug resistance in poultry production: current status and innovative strategies for bacterial control. Microorganisms 2023, 11 (4), 953. [Google Scholar] [CrossRef]
- Fawaz, M.; Haggag, M. Y.; El-Kasrawy, N. A. Applications of nanoparticles of zinc oxide on productive performance of laying hens. SVU-Int. J. Agric. Sci. 2019, 1 (1), 34-45. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, J.; Lu, W.; Zhao, Y.; Zhao, D. Dietary supplementation with different alternatives to in-feed antibiotic improves growth performance of broilers during specific phases. Poult. Sci. 2023, 102 (10), 102919. [Google Scholar] [CrossRef]
- Saleh, A. A.; Paray, B. A.; Dawood, M. A. O.; Abdel-Latif, H. M. R.; Mohammed, H. A.; Shukry, M. Exogenous dietary enzyme formulations improve growth performance of broiler chickens fed a low-energy diet targeting the intestinal nutrient transporter genes. PLoS One 2018, 13 (5), e0198085. [Google Scholar] [CrossRef]
- Barros, V. R. S. M.; Silva, C. A.; Nunes, R. V.; Rodrigues, P. B.; Sakomura, N. K.; Fernandes, J. B. K. β-Mannanase and mannan oligosaccharides in broiler chicken feed. Ciênc. Rural 2015, 45 (1), 111-117. [Google Scholar] [CrossRef]
- Latorre, J. D.; Hernandez-Velasco, X.; Kallapura, G.; Menconi, A.; Pumford, N. R.; Morgan, M. J.; Layton, S. L.; Bielke, L. R.; Hargis, B. M.; Téllez, G. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 2016, 3, 95. [Google Scholar] [CrossRef]
- Georganas, A.; Tsinas, A.; Koutsidis, G.; Giannenas, I. Utilization of agro-industrial by-products for sustainable poultry production. Sustainability 2023, 15 (4), 3679. [Google Scholar] [CrossRef]
- Yang, W.; Xu, Q.; Xu, H.; Liu, Y.; Zhang, Y. A review on the alternatives to antibiotics and the treatment of antibiotic pollution: current development and future prospects. Sci. Total Environ. 2024, 926, 171757. [Google Scholar] [CrossRef]
- Zeng, M.; van Pijkeren, J. P.; Pan, X. Gluco-oligosaccharides as potential prebiotics: synthesis, purification, structural characterization, and evaluation of prebiotic effect. Compr. Rev. Food Sci. Food Saf. 2023, 22 (4), 2611-2651. [Google Scholar] [CrossRef]
- Ferdous, M. F.; Sarker, Y. A.; Akter, S.; Faruque, M. O.; Doley, S.; Miazi, O. F. Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. Adv. Vet. Anim. Res. 2019, 6 (3), 409-415. [Google Scholar] [CrossRef]
- Elghandour, M. M. M. Y.; Salem, A. Z. M.; Castañeda, C. L.; Camacho, L. M.; Kholif, A. E.; Chagoyán, J. C. V.; Cipriano, M. Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: a review. J. Appl. Microbiol. 2020, 128 (3), 658-674. [Google Scholar] [CrossRef]
- Waqas, M.; Nawaz, H.; Rehman, A. U.; Qamar, M. F.; Aslam, A. Effect of yeast based mannan oligosaccharide (Actigen™) supplementation on growth, carcass characteristics and physiological response in broiler chickens. Indian J. Anim. Res. 2019, 53 (11), 1475-1479. [Google Scholar] [DOI]
- Yaqoob, M. U.; Wang, G.; Wang, M. An updated review on probiotics as an alternative of antibiotics in poultry - a review. Anim. Biosci. 2022, 35 (8), 1109-1120. [Google Scholar] [CrossRef]
- Rabetafika, H. N.; Kuda, T.; Hirayama, K. Probiotics as antibiotic alternatives for human and animal applications. Encyclopedia 2023, 3 (2), 561-581. [Google Scholar] [CrossRef]
- El-Moneim, A. E.-M. E. A.; El-Wardany, I.; El-Wardany, D. M.; El-Bahr, S. M. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob. Proteins 2020, 12 (2), 439-450. [Google Scholar] [CrossRef]
- Abou-Kassem, D. E.; Mahrose, K. M.; Abdelnour, S. A.; Elwan, H. A. M.; Taha, A. E.; El-Naggar, K.; El-Kassas, S. Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum). Poult. Sci. 2021, 100 (1), 84-93. [Google Scholar] [CrossRef]
- Ahmad, R.; Aslam, M.; Ashraf, M. A.; Rafique, M. Probiotics as a friendly antibiotic alternative: assessment of their effects on the health and productive performance of poultry. Fermentation 2022, 8 (12), 672. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, M.; Shi, Y.; An, H.; Wei, H.; Xu, J. Horizontally acquired polysaccharide-synthetic gene cluster from Weissella cibaria boosts the probiotic property of Lactiplantibacillus plantarum. Front. Microbiol. 2021, 12, 692957. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Zhao, D.; Lu, W. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly-hatched chicks. Sci. Rep. 2018, 8 (1), 2229. [Google Scholar] [CrossRef]
- Elmi, V. A.; Morshedi, A.; Mehri, M.; Tangestani, R.; Yazdi, M. H. Effects of Lactobacillus acidophilus and natural antibacterials on growth performance and Salmonella colonization in broiler chickens challenged with Salmonella enteritidis. Livest. Sci. 2020, 233, 103948. [Google Scholar] [CrossRef]
- Olnood, C. G.; Beski, S. S. M.; Choct, M.; Iji, P. A. Use of Lactobacillus johnsonii in broilers challenged with Salmonella sofia. Anim. Nutr. 2015, 1 (3), 203-212. [Google Scholar] [CrossRef]
- Nishiyama, T.; Fujimura, T.; Umeda, S.; Mukai, T. Dietary Bacillus subtilis C-3102 supplementation enhances the exclusion of Salmonella enterica from chickens. J. Poult. Sci. 2021, 58 (2), 138-145. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Liu, H.; Zhang, R. Differential analysis of gut microbiota and the effect of dietary Enterococcus faecium supplementation in broiler breeders with high or low laying performance. Poult. Sci. 2021, 100 (2), 1109-1119. [Google Scholar] [CrossRef]
- Han, G. G.; Kim, E. B.; Choi, Y. J.; Lee, J.; Lee, H. B.; Kim, H. Y.; Chae, B. J.; Kim, J.; Choi, Y. Improved antimicrobial activity of Pediococcus acidilactici against Salmonella Gallinarum by UV mutagenesis and genome shuffling. Appl. Microbiol. Biotechnol. 2017, 101 (13), 5353-5363. [Google Scholar] [CrossRef]
- Kanwal, H.; Anjum, A. A.; Khan, S.; Ali, M. Probiotic characterization and population diversity analysis of gut-associated Pediococcus acidilactici for its potential use in the dairy industry. Appl. Sci. 2021, 11 (20), 9586. [Google Scholar] [CrossRef]
- Penha Filho, R. A. C.; Ferreira, J. C.; Kanashiro, A. M. I.; de Sá, M. E. P.; Lemos, E. G. d. M. Immunomodulatory activity and control of Salmonella Enteritidis colonization in the intestinal tract of chickens by Lactobacillus-based probiotic. Vet. Immunol. Immunopathol. 2015, 167 (1-2), 64-69. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, X.; Li, X.; Shi, L.; Wang, Y.; Han, Y. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult. Sci. 2021, 100 (9), 101323. [Google Scholar] [CrossRef]
- Richards-Rios, P. Understanding the Chicken Intestinal Microbiome: Towards a Rational Approach to Feed-Based Interventions; Ph.D. Dissertation, The University of Liverpool: Liverpool, U.K., 2020. [Google Scholar]
- Zhang, S.; Zhong, G.; Shao, D.; Wang, Q.; Hu, Y.; Wu, T.; Ji, C.; Shi, S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021, 100 (3), 100935. [Google Scholar] [CrossRef]
- Abdelqader, A.; Qarallah, B.; Al-Sharafat, A.; Al-Fataftah, A. R. Probiotic bacteria maintain normal growth mechanisms of heat-stressed broiler chickens. J. Therm. Biol. 2020, 92, 102654. [Google Scholar] [CrossRef]
- Mohammed, A. A.; Jacobs, J. A.; Murugesan, G. R.; Cheng, H. W. Effects of dietary supplementation of a probiotic (Bacillus subtilis) on bone mass and meat quality of broiler chickens. Poult. Sci. 2021, 100 (3), 100906. [Google Scholar] [CrossRef]
- Keerqin, C.; Rhayat, L.; Zhang, Z.; Gharib-Naseri, K.; Kheravii, S. K.; Devillard, E.; Ekmay, R. D.; Wu, S. Probiotic Bacillus subtilis 29,784 improved weight gain and enhanced gut health status of broilers under necrotic enteritis condition. Poult. Sci. 2021, 100 (4), 100981. [Google Scholar] [CrossRef]
- Neijat, M.; Shirley, R. B.; Barton, J.; Thiery, P.; Kiarie, E. Effect of dietary supplementation of Bacillus subtilis DSM29784 on hen performance, egg quality indices, and apparent retention of dietary components in laying hens from 19 to 48 weeks of age. Poult. Sci. 2019, 98 (11), 5622-5635. [Google Scholar] [CrossRef]
- Sharma, M. K.; Khan, N.; Bhat, A. R.; Khan, N. A. Effect of dietary supplementation of probiotic Aspergillus niger on performance and cecal microbiota in Hy-Line W-36 laying hens. Animals 2022, 12 (18), 2406. [Google Scholar] [CrossRef]
- Al-Khalaifah, H. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97 (11), 3807-3815. [Google Scholar] [CrossRef]
- El-Deep, M. H.; Abdeen, A.; Abou-Kassem, D. E.; El-Hack, M. E. A.; Alagawany, M.; Taha, A. E. Aspergillus awamori positively impacts the growth performance, nutrient digestibility, antioxidative activity and immune responses of growing rabbits. Vet. Med. Sci. 2021, 7 (1), 226-235. [Google Scholar] [CrossRef]
- Zahirian, M.; Chamani, M.; Sadeghi, A. A.; Aminafshar, M.; Safaei, M. Dietary supplementation of Aspergillus oryzae meal and its effect on performance, carcass characteristics, blood variables, and immunity of broiler chickens. Trop. Anim. Health Prod. 2019, 51 (8), 2263-2268. [Google Scholar] [CrossRef]
- Montes de Oca, R.; Flores, A.; Mendoza, G.; Pérez, L.; Rodríguez, J. Yeast: Description and structure. In Yeast Additive and Animal Production; PubBioMed Central Research Publishing Services: Tamilnadu, 2016; pp 4–13. [Google Scholar]
- Fathima, S.; Hakeem, W. G. A.; Shanmugam, S.; Selvaraj, R. K.; Patchaikannu, P. Yeasts and yeast-based products in poultry nutrition. J. Appl. Poult. Res. 2023, 32 (2), 100345. [Google Scholar] [CrossRef]
- Oliveira, T.; Ferreira, C.; Silva, S.; Gomes, P.; Peres, C. Probiotic potential of indigenous yeasts isolated during the fermentation of table olives from northeast of Portugal. Innov. Food Sci. Emerg. Technol. 2017, 44, 167–172. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, H.; Li, X.; Zhang, Y.; Liu, J. Yeast probiotic and yeast products in enhancing livestock feeds utilization and performance: An overview. J. Fungi 2022, 8 (11), 1191. [Google Scholar] [CrossRef]
- Moonsamy, G.; Govender, P.; Dube, Z.; Mthiyane, T.; Khumalo, Z. Advances in yeast probiotic production and formulation for preventative health. Microorganisms 2024, 12 (11), 2233. [Google Scholar] [CrossRef]
- Abd El-Ghany, W. A.; Shaalan, M.; Salem, H. M. Nanoparticles applications in poultry production: An updated review. World's Poult. Sci. J. 2021, 77 (4), 1001–1025. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Rahman, A.; Mohamad, R.; Zaidan, U. H.; Samsudin, A. A. Antibacterial potential of biosynthesized zinc oxide nanoparticles against poultry-associated foodborne pathogens: An in vitro study. Animals 2021, 11 (7), 2093. [Google Scholar] [CrossRef]
- Chakraborty, N.; Banerjee, J.; Chakraborty, P.; Patra, S.; Ghosh, A. Nanobiotics against antimicrobial resistance: Harnessing the power of nanoscale materials and technologies. J. Nanobiotechnol. 2022, 20 (1), 375. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Y.; Zhou, L.; Lu, H.; Zheng, X. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Yang, J.; Li, W.; Chen, L. Fe₃O₄ nanoparticles attenuated Salmonella infection in chicken liver through reactive oxygen and autophagy via PI3K/Akt/mTOR signaling. Front. Physiol. 2020, 10, 1580. [Google Scholar] [CrossRef]
- Ali, N. M.; Bakheet, A. Evaluation of the inhibitory effect of chitosan nanoparticles on biofilm forming Escherichia coli isolated from omphalitis cases. J. Adv. Vet. Res. 2020, 10 (4), 213–218. [Google Scholar]
- Abd El-Ghany, W. Nanotechnology and its considerations in poultry field: An overview. J. Hellenic Vet. Med. Soc. 2019, 70 (3), 1611–1616. [Google Scholar] [CrossRef]
- Tee, J. K.; Setyawati, M. I.; Peng, F.; Leong, D. T.; Ho, H. K. Oxidative stress by inorganic nanoparticles. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2016, 8 (3), 414–438. [Google Scholar] [CrossRef]
- Pushparaj, S. S. C.; Gopal, J.; Muthu, M. Surveying the resilience of novel metal oxide nanoparticle-based antibiotics—Future scope and direction. Biomass Convers. Biorefin. 2024, 14 (23), 29249–29263. [Google Scholar] [CrossRef]
- Ayoubi, M.; Kamali, R.; Bagherzadeh, M.; Amini, S. M.; Ghahremani, F. Biochemical mechanisms of dose-dependent cytotoxicity and ROS-mediated apoptosis induced by lead sulfide/graphene oxide quantum dots for potential bioimaging applications. Sci. Rep. 2017, 7 (1), 12896. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E. Molecular aspects of the functioning of pathogenic bacteria biofilm based on quorum sensing (QS) signal-response system and innovative non-antibiotic strategies for their elimination. Int. J. Mol. Sci. 2024, 25 (5), 2655. [Google Scholar] [CrossRef]
- Lade, H.; Paul, D.; Kweon, J. H. N-acyl homoserine lactone-mediated quorum sensing with special reference to use of quorum quenching bacteria in membrane biofouling control. BioMed Res. Int. 2014, 2014 (1), 162584. [Google Scholar] [CrossRef]
- Ahsan, A.; Khan, M. A.; Farooq, M. A.; Ahmad, I.; Alotaibi, M. O. Lipid nanocarriers-enabled delivery of antibiotics and antimicrobial adjuvants to overcome bacterial biofilms. Pharmaceutics 2024, 16 (3), 396. [Google Scholar] [CrossRef]
- Pinilla, C. M. B.; Lopes, N. A.; Brandelli, A. Lipid-based nanostructures for the delivery of natural antimicrobials. Molecules 2021, 26 (12), 3587. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L. Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. J. Water Health 2018, 16 (2), 171–180. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Biswal, S. K.; Pradhan, N.; Panda, D.; Mishra, B. K. Biofilm inhibition on medical devices and implants using carbon dots: An updated review. ACS Appl. Bio Mater. 2024, 7 (5), 2604–2619. [Google Scholar] [CrossRef]
- Berger, I.; Loewy, Z. G. Antimicrobial resistance and novel alternative approaches to conventional antibiotics. Bacteria 2024, 3 (3), 171–182. [Google Scholar] [CrossRef]
- Garvey, M. Bacteriophages and the One Health approach to combat multidrug resistance: Is this the way? Antibiotics 2020, 9 (7), 414. [Google Scholar] [CrossRef]
- Robles Ramirez, O.; Sánchez López, J. D.; García Contreras, R.; Martínez de la Peña, C. F. Antimicrobial peptides in livestock: A review with a One Health approach. Front. Cell. Infect. Microbiol. 2024, 14, 1339285. [Google Scholar] [CrossRef]
- Franklin, A. M.; Williams-Nguyen, J.; Paulk, C.; Boxall, A. B. A. A One Health approach for monitoring antimicrobial resistance: Developing a national freshwater pilot effort. Front. Water 2024, 6, 1359109. [Google Scholar] [CrossRef]
- Walker, H.; Bedford, M.; Cowieson, A.; Olukosi, O. The effect of including a mixed-enzyme product in broiler diets on performance, metabolizable energy, phosphorus and calcium retention. Animals 2024, 14 (2), 328. [Google Scholar] [CrossRef]
- Vilela, D.; Stanton, M. M.; Parmar, J.; Sánchez, S. Drug-free enzyme-based bactericidal nanomotors against pathogenic bacteria. ACS Appl. Mater. Interfaces 2021, 13 (13), 14964–14973. [Google Scholar] [CrossRef]
- Alaoui Mdarhri, H.; Benmessaoud, R.; Bouymajane, A.; Filali, F. R. Alternatives therapeutic approaches to conventional antibiotics: Advantages, limitations and potential application in medicine. Antibiotics 2022, 11 (12), 1826. [Google Scholar] [CrossRef]
- McAllister, T. A.; Topp, E.; Gow, S.; Hannon, S. J.; Alexander, T. Challenges of a One Health approach to the development of alternatives to antibiotics. Anim. Front. 2018, 8 (2), 10–20. [Google Scholar] [CrossRef]
- Ioannou, E.; Labrou, N. E. Development of enzyme-based cosmeceuticals: Studies on the proteolytic activity of Arthrospira platensis and its efficient incorporation in a hydrogel formulation. Cosmetics 2022, 9 (5), 106. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X. Effect of lactic acid bacteria fermentation on plant-based products. Fermentation 2024, 10 (1), 48. [Google Scholar] [CrossRef]
- Ismail, H.; Khalifa, E.; El-Hack, M. E. A.; Alagawany, M.; Swelum, A. A. Prospective application of nanoencapsulated Bacillus amyloliquefaciens on broiler chickens' performance and gut health with efficacy against Campylobacter jejuni colonization. Animals 2023, 13 (5), 775. [Google Scholar] [CrossRef]
- Gheorghe-Irimia, R.-A.; Tăpăloagă, D.; Tăpăloagă, P. R.; Ghimpețeanu, O. M. Exploring the synergy: Essential oils in animal nutrition and their role in enhancing production. Ann. Univ. Craiova, Agric. Montanol. Cadastre Ser. 2023, 53 (1), 141–148. [Google Scholar]
Publisher’s Note: The views expressed in all publications, including statements, opinions, and data, are solely those of the individual author(s) and contributor(s) and do not necessarily reflect the views of Insights Academic Publishing (IAP) and/or its editor(s). IAP and/or its editor(s) are not responsible for any injury to persons or damage to property resulting from the ideas, methods, instructions, or products referenced in the content.
Copyright: © 2025 by the authors.
License: This article is published under the Creative Commons Attribution 4.0 International.CC BY 4.0
Publisher: Insights Academic Publishing (IAP), Lahore, Pakistan.

